Hes1 regulates anagen initiation and hair follicle regeneration through modulation of hedgehog signaling
- PMID: 31721388
- PMCID: PMC7027765
- DOI: 10.1002/stem.3117
Hes1 regulates anagen initiation and hair follicle regeneration through modulation of hedgehog signaling
Abstract
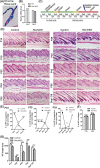
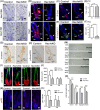
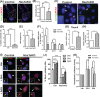
Adult hair follicles undergo repeated cycling of regression (catagen), resting (telogen), and growth (anagen), which is maintained by hair follicle stem cells (HFSCs). The mechanism underlying hair growth initiation and HFSC maintenance is not fully understood. Here, by epithelial deletion of Hes1, a major Notch downstream transcriptional repressor, we found that hair growth is retarded, but the hair cycle progresses normally. Hes1 is specifically upregulated in the lower bulge/HG during anagen initiation. Accordingly, loss of Hes1 results in delayed activation of the secondary hair germ (HG) and shortened anagen phase. This developmental delay causes reduced hair shaft length but not identity changes in follicular lineages. Remarkably, Hes1 ablation results in impaired hair regeneration upon repetitive depilation. Microarray gene profiling on HFSCs indicates that Hes1 modulates Shh responsiveness in anagen initiation. Using primary keratinocyte cultures, we demonstrated that Hes1 deletion negatively influences ciliogenesis and Smoothened ciliary accumulation upon Shh treatment. Furthermore, transient application of Smoothened agonist during repetitive depilation can rescue anagen initiation and HFSC self-renewal in Hes1-deficient hair follicles. We reveal a critical function of Hes1 in potentiating Shh signaling in anagen initiation, which allows sufficient signaling strength to expand the HG and replenish HFSCs to maintain the hair cycle homeostasis.
Keywords: adult stem cells; cellular proliferation; epidermis; notch; signal transduction.
©2019 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2019.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

