Regulating ENaC's gate
- PMID: 31721612
- PMCID: PMC6985836
- DOI: 10.1152/ajpcell.00418.2019
Regulating ENaC's gate
Abstract
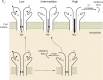
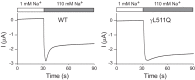
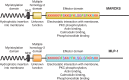
Epithelial Na+ channels (ENaCs) are members of a family of cation channels that function as sensors of the extracellular environment. ENaCs are activated by specific proteases in the biosynthetic pathway and at the cell surface and remove embedded inhibitory tracts, which allows channels to transition to higher open-probability states. Resolved structures of ENaC and an acid-sensing ion channel revealed highly organized extracellular regions. Within the periphery of ENaC subunits are unique domains formed by antiparallel β-strands containing the inhibitory tracts and protease cleavage sites. ENaCs are inhibited by Na+ binding to specific extracellular site(s), which promotes channel transition to a lower open-probability state. Specific inositol phospholipids and channel modification by Cys-palmitoylation enhance channel open probability. How these regulatory factors interact in a concerted manner to influence channel open probability is an important question that has not been resolved. These various factors are reviewed, and the impact of specific factors on human disorders is discussed.
Keywords: ASIC; ENaC; gating; palmitoylation; phosphatidylinositol; protease; sodium.
Conflict of interest statement
T. R. Kleyman receives an honorarium from Wiley, Inc., as Editor-in-Chief of
Figures


References
-
- Alli AA, Bao HF, Liu BC, Yu L, Aldrugh S, Montgomery DS, Ma HP, Eaton DC. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol 309: F456–F463, 2015. doi:10.1152/ajprenal.00631.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

