Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth: Findings from Mendelian randomization and parental negative control studies
- PMID: 31721775
- PMCID: PMC6853297
- DOI: 10.1371/journal.pmed.1002972
Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth: Findings from Mendelian randomization and parental negative control studies
Abstract
Background: Maternal smoking during pregnancy is an established risk factor for low infant birth weight, but evidence on critical exposure windows and timing of fetal growth restriction is limited. Here we investigate the associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth by triangulating evidence from 3 analytical approaches to strengthen causal inference.
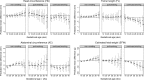
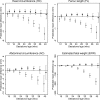
Methods and findings: We analysed data from 8,621 European liveborn singletons in 2 population-based pregnancy cohorts (the Generation R Study, the Netherlands 2002-2006 [n = 4,682]) and the Born in Bradford study, United Kingdom 2007-2010 [n = 3,939]) with fetal ultrasound and birth anthropometric measures, parental smoking during pregnancy, and maternal genetic data. Associations with trajectories of estimated fetal weight (EFW) and individual fetal parameters (head circumference, femur length [FL], and abdominal circumference [AC]) from 12-16 to 40 weeks' gestation were analysed using multilevel fractional polynomial models. We compared results from (1) confounder-adjusted multivariable analyses, (2) a Mendelian randomization (MR) analysis using maternal rs1051730 genotype as an instrument for smoking quantity and ease of quitting, and (3) a negative control analysis comparing maternal and mother's partner's smoking associations. In multivariable analyses, women who continued smoking during pregnancy had a smaller fetal size than non-smokers from early gestation (16-20 weeks) through to birth (p-value for each parameter < 0.001). Fetal size reductions in continuing smokers followed a dose-dependent pattern (compared to non-smokers, difference in mean EFW [95% CI] at 40 weeks' gestation was -144 g [-182 to -106], -215 g [-248 to -182], and -290 g [-334 to -247] for light, moderate, and heavy smoking, respectively). Overall, fetal size reductions were most pronounced for FL. The fetal growth trajectory in women who quit smoking in early pregnancy was similar to that of non-smokers, except for a shorter FL and greater AC around 36-40 weeks' gestation. In MR analyses, each genetically determined 1-cigarette-per-day increase was associated with a smaller EFW from 20 weeks' gestation to birth in smokers (p = 0.01, difference in mean EFW at 40 weeks = -45 g [95% CI -81 to -10]) and a greater EFW from 32 weeks' gestation onwards in non-smokers (p = 0.03, difference in mean EFW at 40 weeks = 26 g [95% CI 5 to 47]). There was no evidence that partner smoking was associated with fetal growth. Study limitations include measurement error due to maternal self-report of smoking and the modest sample size for MR analyses resulting in unconfounded estimates being less precise. The apparent positive association of the genetic instrument with fetal growth in non-smokers suggests that genetic pleiotropy may have masked a stronger association in smokers.
Conclusions: A consistent linear dose-dependent association of maternal smoking with fetal growth was observed from the early second trimester onwards, while no major growth deficit was found in women who quit smoking early in pregnancy except for a shorter FL during late gestation. These findings reinforce the importance of smoking cessation advice in preconception and antenatal care and show that smoking reduction can lower the risk of impaired fetal growth in women who struggle to quit.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: DAL has received support from several national and international government and charitable funders, and from Medtronic Ltd and Roche Diagnostics for research unrelated to that presented here. The other authors report no conflicts.
Figures


References
-
- Office of the Surgeon General, Office on Smoking and Health. The health consequences of smoking: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 2004. - PubMed
-
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. How tobacco smoke causes disease—the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General Atlanta: Centers for Disease Control and Prevention; 2010. - PubMed
-
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA. 1984;251:911–5. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/K021656/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/3/MRC_/Medical Research Council/United Kingdom
- AA/18/7/34219/BHF_/British Heart Foundation/United Kingdom
- CS/16/4/32482/BHF_/British Heart Foundation/United Kingdom
- G0601712/MRC_/Medical Research Council/United Kingdom
- CS/16/4/32482 /BHF_/British Heart Foundation/United Kingdom
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/N024397/1/MRC_/Medical Research Council/United Kingdom
- WT101597MA/WT_/Wellcome Trust/United Kingdom
- NF-SI-0611-10196/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

