Equine bronchial fibroblasts enhance proliferation and differentiation of primary equine bronchial epithelial cells co-cultured under air-liquid interface
- PMID: 31721813
- PMCID: PMC6853605
- DOI: 10.1371/journal.pone.0225025
Equine bronchial fibroblasts enhance proliferation and differentiation of primary equine bronchial epithelial cells co-cultured under air-liquid interface
Abstract
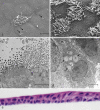
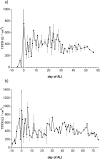
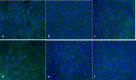
Interaction between epithelial cells and fibroblasts play a key role in wound repair and remodelling in the asthmatic airway epithelium. We present the establishment of a co-culture model using primary equine bronchial epithelial cells (EBECs) and equine bronchial fibroblasts (EBFs). EBFs at passage between 4 and 8 were seeded on the bottom of 24-well plates and treated with mitomycin C at 80% confluency. Then, freshly isolated (P0) or passaged (P1) EBECs were seeded on the upper surface of membrane inserts that had been placed inside the EBF-containing well plates and grown first under liquid-liquid interface (LLI) then under air-liquid interface (ALI) conditions to induce epithelial differentiation. Morphological, structural and functional markers were monitored in co-cultured P0 and P1 EBEC monolayers by phase-contrast microscopy, scanning and transmission electron microscopy, hematoxylin-eosin, immunocytochemistry as well as by measuring the transepithelial electrical resistance (TEER) and transepithelial transport of selected drugs. After about 15-20 days of co-culture at ALI, P0 and P1 EBEC monolayers showed pseudo-stratified architecture, presence of ciliated cells, typically honeycomb-like pattern of tight junction protein 1 (TJP1) expression, and intact selective barrier functions. Interestingly, some notable differences were observed in the behaviour of co-cultured EBECs (adhesion to culture support, growth rate, differentiation rate) as compared to our previously described EBEC mono-culture system, suggesting that cross-talk between epithelial cells and fibroblasts actually takes place in our current co-culture setup through paracrine signalling. The EBEC-EBF co-culture model described herein will offer the opportunity to investigate epithelial-mesenchymal cell interactions and underlying disease mechanisms in the equine airways, thereby leading to a better understanding of their relevance to pathophysiology and treatment of equine and human asthma.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 1999; 29 Suppl. 2: 90–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

