Fc-modified HIT-like monoclonal antibody as a novel treatment for sepsis
- PMID: 31722003
- PMCID: PMC7059515
- DOI: 10.1182/blood.2019002329
Fc-modified HIT-like monoclonal antibody as a novel treatment for sepsis
Abstract
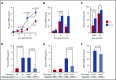
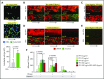
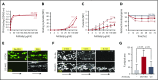
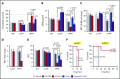
Sepsis is characterized by multiorgan system dysfunction that occurs because of infection. It is associated with high morbidity and mortality and is in need of improved therapeutic interventions. Neutrophils play a crucial role in sepsis, releasing neutrophil extracellular traps (NETs) composed of DNA complexed with histones and toxic antimicrobial proteins that ensnare pathogens, but also damage host tissues. At presentation, patients often have a significant NET burden contributing to the multiorgan damage. Therefore, interventions that inhibit NET release would likely be ineffective at preventing NET-based injury. Treatments that enhance NET degradation may liberate captured bacteria and toxic NET degradation products (NDPs) and likely be of limited therapeutic benefit as well. We propose that interventions that stabilize NETs and sequester NDPs may be protective in sepsis. We showed that platelet factor 4 (PF4), a platelet-associated chemokine, binds and compacts NETs, increasing their resistance to DNase I. We now show that PF4 increases NET-mediated bacterial capture, reduces the release of NDPs, and improves outcome in murine models of sepsis. A monoclonal antibody KKO which binds to PF4-NET complexes, further enhances DNase resistance. However, the Fc portion of this antibody activates the immune response and increases thrombotic risk, negating any protective effects in sepsis. Therefore, we developed an Fc-modified KKO that does not induce these negative outcomes. Treatment with this antibody augmented the effects of PF4, decreasing NDP release and bacterial dissemination and increasing survival in murine sepsis models, supporting a novel NET-targeting approach to improve outcomes in sepsis.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

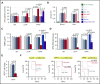
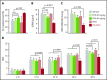
Comment in
-
"HIT"ing back against NETs.Blood. 2020 Mar 5;135(10):706-707. doi: 10.1182/blood.2019004461. Blood. 2020. PMID: 32135015 No abstract available.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. - PubMed
-
- Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906-918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

