Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer
- PMID: 31722153
- PMCID: PMC6941470
- DOI: 10.1056/NEJMoa1902626
Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer
Abstract
Background: Secondary surgical cytoreduction in women with platinum-sensitive, recurrent epithelial ovarian, primary peritoneal, or fallopian-tube ("ovarian") cancer is widely practiced but has not been evaluated in phase 3 investigation.
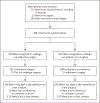
Methods: We randomly assigned patients with recurrent ovarian cancer who had received one previous therapy, had an interval during which no platinum-based chemotherapy was used (platinum-free interval) of 6 months or more, and had investigator-determined resectable disease (to no macroscopic residual disease) to undergo secondary surgical cytoreduction and then receive platinum-based chemotherapy or to receive platinum-based chemotherapy alone. Adjuvant chemotherapy (paclitaxel-carboplatin or gemcitabine-carboplatin) and use of bevacizumab were at the discretion of the investigator. The primary end point was overall survival.
Results: A total of 485 patients underwent randomization, 240 to secondary cytoreduction before chemotherapy and 245 to chemotherapy alone. The median follow-up was 48.1 months. Complete gross resection was achieved in 67% of the patients assigned to surgery who underwent the procedure. Platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance was administered to 84% of the patients overall and was equally distributed between the two groups. The hazard ratio for death (surgery vs. no surgery) was 1.29 (95% confidence interval [CI], 0.97 to 1.72; P = 0.08), which corresponded to a median overall survival of 50.6 months and 64.7 months, respectively. Adjustment for platinum-free interval and chemotherapy choice did not alter the effect. The hazard ratio for disease progression or death (surgery vs. no surgery) was 0.82 (95% CI, 0.66 to 1.01; median progression-free survival, 18.9 months and 16.2 months, respectively). Surgical morbidity at 30 days was 9%; 1 patient (0.4%) died from postoperative complications. Patient-reported quality of life decreased significantly after surgery but did not differ significantly between the two groups after recovery.
Conclusions: In this trial involving patients with platinum-sensitive, recurrent ovarian cancer, secondary surgical cytoreduction followed by chemotherapy did not result in longer overall survival than chemotherapy alone. (Funded by the National Cancer Institute and others; GOG-0213 ClinicalTrials.gov number, NCT00565851.).
Copyright © 2019 Massachusetts Medical Society.
Figures



Comment in
-
Prospective evidence discourages secondary cytoreductive surgery.Nat Rev Clin Oncol. 2020 Feb;17(2):68. doi: 10.1038/s41571-019-0309-y. Nat Rev Clin Oncol. 2020. PMID: 31804614 No abstract available.
-
Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer.N Engl J Med. 2020 Feb 13;382(7):685. doi: 10.1056/NEJMc1916477. N Engl J Med. 2020. PMID: 32053312 No abstract available.
-
Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer.N Engl J Med. 2020 Feb 13;382(7):685-686. doi: 10.1056/NEJMc1916477. N Engl J Med. 2020. PMID: 32053313 No abstract available.
-
Secondary surgical cytoreduction needs to be assessed taking into account surgical technique, completeness of cytoreduction, and extent of disease.World J Surg Oncol. 2020 May 11;18(1):92. doi: 10.1186/s12957-020-01853-4. World J Surg Oncol. 2020. PMID: 32393274 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69: 7–34. - PubMed
-
- Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 2009;112: 265–74. - PubMed
-
- Bristow RE, Lagasse LD, Karlan BY. Secondary surgical cytoreduction for advanced epithelial ovarian cancer: patient selection and review of the literature. Cancer 1996;78:2049–62. - PubMed
-
- Skipper HE. Adjuvant chemotherapy. Cancer 1978;41:936–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- CA 37517/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10CA180868/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- 1U10 CA180822/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- CA 27469/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- P30CA016672/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- UG1 CA233193/CA/NCI NIH HHS/United States
- U10 CA037517/CA/NCI NIH HHS/United States
