Sex Differences in the Blood Transcriptome Identify Robust Changes in Immune Cell Proportions with Aging and Influenza Infection
- PMID: 31722210
- PMCID: PMC6856718
- DOI: 10.1016/j.celrep.2019.10.019
Sex Differences in the Blood Transcriptome Identify Robust Changes in Immune Cell Proportions with Aging and Influenza Infection
Abstract
Sex differences in autoimmunity and infection suggest that a better understanding of molecular sex differences will improve the diagnosis and treatment of immune-related disease. We identified 144 differentially expressed genes, referred to as immune sex expression signature (iSEXS), between human males and females using an integrated multi-cohort analysis of blood transcriptome profiles from six discovery cohorts from five continents with 458 healthy individuals. We validated iSEXS in 11 additional cohorts of 524 peripheral blood samples. When we separated iSEXS into genes located on sex chromosomes (XY-iSEXS) or autosomes (autosomal-iSEXS), both modules distinguished males and females. iSEXS reflects sex differences in immune cell proportions, with female-associated genes showing higher expression by CD4+ T cells and male-associated genes showing higher expression by myeloid cells. Autosomal-iSEXS detected an increase in monocytes with age in females, reflected sex-differential immune cell dynamics during influenza infection, and predicted antibody response in males, but not females.
Keywords: CD4(+) T cells; aging; immune system; immunology; influenza; meta-analysis; monocytes; multi-cohort analysis; sex differences; transcriptome.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





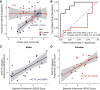
References
-
- Ben-Hur H., Mor G., Insler V., Blickstein I., Amir-Zaltsman Y., Sharp A., Globerson A., Kohen F. Menopause is associated with a significant increase in blood monocyte number and a relative decrease in the expression of estrogen receptors in human peripheral monocytes. Am. J. Reprod. Immunol. 1995;34:363–369. - PubMed
-
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Stat. Methodol. 1995;57:289–300.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

