RNA sequencing reveals novel macrophage transcriptome favoring neurovascular plasticity after ischemic stroke
- PMID: 31722596
- PMCID: PMC7168800
- DOI: 10.1177/0271678X19888630
RNA sequencing reveals novel macrophage transcriptome favoring neurovascular plasticity after ischemic stroke
Abstract
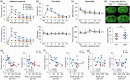
Blood monocytes/macrophages infiltrate the brain after ischemic stroke and critically influence brain injury and regeneration. We investigated stroke-induced transcriptomic changes of monocytes/macrophages by RNA sequencing profiling, using a mouse model of permanent focal cerebral ischemia. Compared to non-ischemic conditions, brain ischemia induced only moderate genomic changes in blood monocytes, but triggered robust genomic reprogramming in monocytes/macrophages invading the brain. Surprisingly, functional enrichment analysis of the transcriptome of brain macrophages revealed significant overrepresentation of biological processes linked to neurovascular remodeling, such as angiogenesis and axonal regeneration, as early as five days after stroke, suggesting a previously underappreciated role for macrophages in initiating post-stroke brain repair. Upstream Regulator analysis predicted peroxisome proliferator-activated receptor gamma (PPARγ) as a master regulator driving the transcriptional reprogramming in post-stroke brain macrophages. Importantly, myeloid cell-specific PPARγ knockout (mKO) mice demonstrated lower post-stroke angiogenesis and neurogenesis than wild-type mice, which correlated significantly with the exacerbation of post-stroke neurological deficits in mKO mice. Collectively, our findings reveal a novel repair-enhancing transcriptome in brain macrophages during post-stroke neurovascular remodeling. As a master switch controlling genomic reprogramming, PPARγ is a rational therapeutic target for promoting and maintaining beneficial macrophage functions, facilitating neurorestoration, and improving long-term functional recovery after ischemic stroke.
Keywords: Angiogenesis; PPARγ; fluorescence-activated cell sorting; focal cerebral ischemia; neurogenesis.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

