The mitochondrial calcium uniporter is crucial for the generation of fast cortical network rhythms
- PMID: 31722597
- PMCID: PMC7585921
- DOI: 10.1177/0271678X19887777
The mitochondrial calcium uniporter is crucial for the generation of fast cortical network rhythms
Abstract
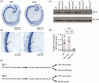
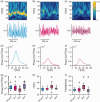
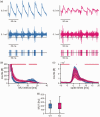
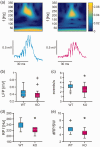
The role of the mitochondrial calcium uniporter (MCU) gene (Mcu) in cellular energy homeostasis and generation of electrical brain rhythms is widely unknown. We investigated this issue in mice and rats using Mcu-knockout and -knockdown strategies in vivo and in situ and determined the effects of these genetic manipulations on hippocampal gamma oscillations (30-70 Hz) and sharp wave-ripples. These physiological network states require precise neurotransmission between pyramidal cells and inhibitory interneurons, support spike-timing and synaptic plasticity and are associated with perception, attention and memory. Absence of the MCU resulted in (i) gamma oscillations with decreased power (by >40%) and lower synchrony, including less precise neural action potential generation ('spiking'), (ii) sharp waves with decreased incidence (by about 22%) and decreased fast ripple frequency (by about 3%) and (iii) lack of activity-dependent pyruvate dehydrogenase dephosphorylation. However, compensatory adaptation in gene expression related to mitochondrial function and glucose metabolism was not detected. These data suggest that the neuronal MCU is crucial for the generation of network rhythms, most likely by influences on oxidative phosphorylation and perhaps by controlling cytoplasmic Ca2+ homeostasis. This work contributes to an increased understanding of mitochondrial Ca2+ uptake in cortical information processing underlying cognition and behaviour.
Keywords: Calcium signalling; electrophysiology; mitochondria; neurometabolic coupling; neuronal activity.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Kann O, Kovács R.Mitochondria and neuronal activity. Am J Physiol Cell Physiol 2007; 292: C641–C657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

