circLMTK2 acts as a sponge of miR-150-5p and promotes proliferation and metastasis in gastric cancer
- PMID: 31722712
- PMCID: PMC6854648
- DOI: 10.1186/s12943-019-1081-4
circLMTK2 acts as a sponge of miR-150-5p and promotes proliferation and metastasis in gastric cancer
Abstract
Background: As a novel class of non-coding RNAs, circular RNAs (circRNAs) are key regulators of the development and progression of different cancers. However, little is known about the function and biological mechanism of circLMTK2, also named hsa_circ_0001725, in gastric cancer (GC) tumourigenesis.
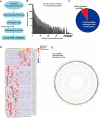
Methods: circLMTK2 was identified in ten paired cancer specimens and adjacent normal tissues by RNA sequencing and genome-wide bioinformatic analysis and verified by quantitative real-time PCR (qRT-PCR). Knockdown or exogenous expression of circLMTK2 combined with in vitro and in vivo assays were performed to prove the functional significance of circLMTK2. The molecular mechanism of circLMTK2 was demonstrated by searching the CircNet database and confirmed by RNA in vivo precipitation assays, western blotting, luciferase assays and rescue experiments.
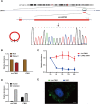
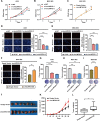
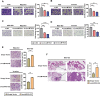
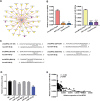
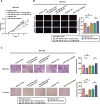
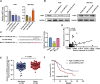
Results: circLMTK2 was frequently upregulated in GC tissues, and high circLMTK2 expression was associated with poor prognosis, lymph node metastasis and poor TNM stage in GC patients. Functionally, circLMTK2 overexpression promoted GC cell proliferation and tumourigenicity in vitro and in vivo. Furthermore, ectopic circLMTK2 expression enhanced GC cell migration and invasion in vitro and tumour metastasis in vivo. In addition, we demonstrated that circLMTK2 could sponge miR-150-5p, thus indirectly regulating the c-Myc expression and contributing to GC tumourigenesis.
Conclusion: Our findings demonstrate that circLMTK2 functions as a tumour promoter in GC through the miR-150-5p/c-Myc axis and could thus be a prognostic predictor and therapeutic target for GC.
Keywords: C-Myc; Gastric cancer; circLMTK2; miR-150-5p.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Hsa_circ_0008667 promotes progression and improves the prognosis of gastric cancer by inhibiting miR-9-5p.Arab J Gastroenterol. 2024 Nov;25(4):349-355. doi: 10.1016/j.ajg.2024.09.002. Epub 2024 Oct 24. Arab J Gastroenterol. 2024. PMID: 39455349
-
Circular RNA hsa_circ_0001829 promotes gastric cancer progression through miR-155-5p/SMAD2 axis.J Exp Clin Cancer Res. 2020 Dec 11;39(1):280. doi: 10.1186/s13046-020-01790-w. J Exp Clin Cancer Res. 2020. Retraction in: J Exp Clin Cancer Res. 2023 Apr 26;42(1):101. doi: 10.1186/s13046-023-02673-6. PMID: 33308284 Free PMC article. Retracted.
-
Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression.Mol Cancer. 2017 Sep 11;16(1):151. doi: 10.1186/s12943-017-0719-3. Mol Cancer. 2017. PMID: 28893265 Free PMC article.
-
Contribution of circRNAs in gastric cancer.Pathol Res Pract. 2021 Nov;227:153640. doi: 10.1016/j.prp.2021.153640. Epub 2021 Sep 28. Pathol Res Pract. 2021. PMID: 34624593 Review.
-
Circular RNAs as important players in human gastric cancer.Clin Transl Oncol. 2021 Jan;23(1):10-21. doi: 10.1007/s12094-020-02419-2. Epub 2020 Jun 25. Clin Transl Oncol. 2021. PMID: 32583185 Review.
Cited by
-
Circular RNAs as diagnostic biomarkers for gastric cancer: A comprehensive update from emerging functions to clinical significances.Front Genet. 2022 Oct 28;13:1037120. doi: 10.3389/fgene.2022.1037120. eCollection 2022. Front Genet. 2022. PMID: 36386850 Free PMC article. Review.
-
Circular RNA hsa_circ_0007367 promotes the progression of pancreatic ductal adenocarcinoma by sponging miR-6820-3p and upregulating YAP1 expression.Cell Death Dis. 2022 Aug 25;13(8):736. doi: 10.1038/s41419-022-05188-8. Cell Death Dis. 2022. PMID: 36008392 Free PMC article.
-
CircAKT3 promotes cell proliferation, survival and glutamine metabolism of gastric cancer by activating SLC1A5 expression via targeting miR-515-5p.Histol Histopathol. 2022 Mar;37(3):227-241. doi: 10.14670/HH-18-401. Epub 2021 Dec 1. Histol Histopathol. 2022. PMID: 34850965
-
Bioinformatics Analysis and Functional Verification of ADAMTS9-AS1/AS2 in Lung Adenocarcinoma.Front Oncol. 2021 Jul 29;11:681777. doi: 10.3389/fonc.2021.681777. eCollection 2021. Front Oncol. 2021. PMID: 34395250 Free PMC article.
-
Circular RNA AKT3 governs malignant behaviors of esophageal cancer cells by sponging miR-17-5p.World J Gastroenterol. 2021 Jan 21;27(3):240-254. doi: 10.3748/wjg.v27.i3.240. World J Gastroenterol. 2021. PMID: 33519139 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

