Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study
- PMID: 31722735
- PMCID: PMC6854774
- DOI: 10.1186/s40425-019-0800-0
Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study
Abstract
Background: Uveal melanoma (UM) is highly refractory to treatment with dismal prognosis in advanced stages. The value of the combined checkpoint blockade with CTLA-4 and PD-1 inhibition in metastatic UM is currently unclear.
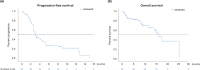
Methods: Patients with metastatic or unresectable UM treated with ipilimumab in combination with a PD-1 inhibitor were collected from 16 German skin cancer centers. Patient records of 64 cases were analyzed for response, progression-free survival (PFS), overall survival (OS), and safety. Clinical parameters and serum biomarkers associated with OS and treatment response were determined with Cox regression modelling and logistic regression.
Results: The best overall response rate to combined checkpoint blockade was 15.6% with 3.1 and 12.5% complete and partial response, respectively. The median duration of response was 25.5 months (range 9.0-65.0). Stable disease was achieved in 21.9%, resulting in a disease control rate of 37.5% with a median duration of the clinical benefit of 28.0 months (range 7.0-65.0). The median PFS was 3.0 months (95% CI 2.4-3.6). The median OS was estimated to 16.1 months (95% CI 12.9-19.3). Regarding safety, 39.1% of treated patients experienced a severe, treatment-related adverse event according to the CTCAE criteria (grade 3: 37.5%; grade 4: 1.6%). The most common toxicities were colitis (20.3%), hepatitis (20.3%), thyreoiditis (15.6%), and hypophysitis (7.8%). A poor ECOG performance status was an independent risk factor for decreased OS (p = 0.007).
Conclusions: The tolerability of the combined checkpoint blockade in UM may possibly be better than in trials on cutaneous melanoma. This study implies that combined checkpoint blockade represents the hitherto most effective treatment option available for metastatic UM available outside of clinical trials.
Keywords: Biomarker; Combined immune checkpoint blockade; Ipilimumab; Nivolumab; Uveal melanoma.
Conflict of interest statement
Teresa Amaral: grants from Neracare; travel support from Novartis, personal fees and travel support from BMS, outside the submitted work; Carola Berking: speaker’s honoraria from BMS, Immunocore, MSD, Novartis, and Roche, consultant’s honoraria from Amgen, BMS, MSD, Novartis, Pierre Fabre, Roche, and Sanofi-Aventis and travel support from Amgen, BMS, MSD, and Roche; Anja Gesierich: speaker’s honoraria from Bristol-Myers Squibb, MSD Sharp & Dohme and Roche, consultant’s honoraria from Amgen, Bristol-Myers Squibb, Novartis, MSD Sharp & Dohme, Pierre Fabre Pharmaceuticals, Pfizer, Roche and Sanofi Genzyme, travel support from Bristol-Myers Squibb, MSD Sharp & Dohme, Novartis and Roche; Lucie Heinzerling speaker’s/ consultant’s honoraria from BMS, MSD, Novartis, Roche, Amgen, Pierre Fabre, Sanofi-Aventis, Curevac, research grants to institution from Novartis; Markus V. Heppt: speaker’s honoraria and/or consultant’s honoraria from Roche, Novartis, BMS, MSD and travel support from BMS; Katharina C. Kähler: consultant to Roche, BMS, MSD and received travel grants and speaker fees from Roche, BMS, MSD, Novartis, Amgen; Carmen Loquai: advisory for Roche, Amgen, Novartis, BMS, MSD, Ribological, speaker’s honoraria from Roche, BMS, MSD, Novartis, travel reimbursement from Roche, BMS, MSD, Novartis; Max Schlaak: speaker’s honoraria and/ or consultant’s honoraria from Roche, Novartis, BMS, MSD, Kyowa Kirin, Amgen, Pierre Fabre and travel support from BMS; Henner M. Stege: travel support from Novartis and Roche; Patrick Terheyden: speaker’s honoraria from BMS, Novartis, MSD, Pierre-Fabre, Curevac and Roche, consultant’s honoraria from BMS, Novartis, Pierre-Fabre, Merck Serono, Sanofi und Roche and travel support fom BMS, Pierre-Fabre and Roche; Kai-Martin Thoms: speaker’s honoraria from BMS, MSD, Novartis and Roche, consultant’s honoraria from BMS, MSD, Novartis, Roche and Pierre Fabre and travel support from BMS, Novartis, Roche and Pierre Fabre; Jochen Utikal: advisory board or honoraria and travel support from Amgen, BMS, GSK, LeoPharma, MSD, Novartis, Pierre Fabre, Roche, outside the submitted work; Lisa Zimmer: consultant/ honoraria from Roche, BMS, MSD, Novartis, Sanofi, Pierre Fabre and travel support from MSD, BMS, Amgen, Pierre Fabre, Sanofi and Novartis. The remaining authors declare no conflict of interest.
Figures

References
-
- Mallone S, De Vries E, Guzzo M, Midena E, Verne J, Coebergh JW, Marcos-Gragera R, Ardanaz E, Martinez R, Chirlaque MD, et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48(8):1167–1175. doi: 10.1016/j.ejca.2011.10.004. - DOI - PubMed
-
- Doherty RE, Alfawaz M, Francis J, Lijka-Jones B, Sisley K. Genetics of Uveal Melanoma. In: Scott JF, Gerstenblith MR, editors. Noncutaneous Melanoma. Brisbane: Codon Publications; 2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
