Prospective clinical testing and experimental validation of the Pediatric Sepsis Biomarker Risk Model
- PMID: 31723040
- PMCID: PMC7720682
- DOI: 10.1126/scitranslmed.aax9000
Prospective clinical testing and experimental validation of the Pediatric Sepsis Biomarker Risk Model
Abstract
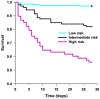
Sepsis remains a major public health problem with no major therapeutic advances over the last several decades. The clinical and biological heterogeneity of sepsis have limited success of potential new therapies. Accordingly, there is considerable interest in developing a precision medicine approach to inform more rational development, testing, and targeting of new therapies. We previously developed the Pediatric Sepsis Biomarker Risk Model (PERSEVERE) to estimate mortality risk and proposed its use as a prognostic enrichment tool in sepsis clinical trials; prognostic enrichment selects patients based on mortality risk independent of treatment. Here, we show that PERSEVERE has excellent performance in a diverse cohort of children with septic shock with potential for use as a predictive enrichment strategy; predictive enrichment selects patients based on likely response to treatment. We demonstrate that the PERSEVERE biomarkers are reliably associated with mortality in mice challenged with experimental sepsis, thus providing an opportunity to test precision medicine strategies in the preclinical setting. Using this model, we tested two clinically feasible therapeutic strategies, guided by the PERSEVERE-based enrichment, and found that mice identified as high risk for mortality had a greater bacterial burden and could be rescued by higher doses of antibiotics. The association between higher pathogen burden and higher mortality risk was corroborated among critically ill children with septic shock. This bedside to bench to bedside approach provides proof of principle for PERSEVERE-guided application of precision medicine in sepsis.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



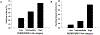
Comment in
-
A Predictive Enrichment Strategy for Precision Medicine in Pediatric Sepsis.Clin Chem. 2020 Apr 1;66(4):629-630. doi: 10.1093/clinchem/hvaa028. Clin Chem. 2020. PMID: 32232454 No abstract available.
References
-
- Cohen J, Vincent J-L, Adhikari NKJ, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Peflrene E, Sepsis: A roadmap for future research. Lancet Infect. Dis 15, 581–614 (2015). - PubMed
-
- Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, Singhi SC, Erickson S, Roy JA, Bush JL, Nadkarni VM, Thomas NJ, Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med 191, 1147–1157 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

