TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway
- PMID: 31723054
- PMCID: PMC6948857
- DOI: 10.1172/jci.insight.129013
TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway
Abstract
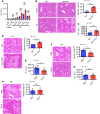
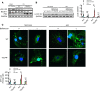
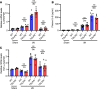
Thymic stromal lymphopoietin (TSLP) is a cytokine mainly released by epithelial cells that plays important roles in inflammation, autoimmune disease, and cancer. While TSLP is expressed in the liver at high levels, the role of TSLP in liver ischemia/reperfusion (I/R) injury remains unknown. Experiments were carried out to determine the role of TSLP in liver I/R injury. Wild-type (WT) and TSLP receptor-knockout (TSLPR-/-) mice were subjected to liver partial warm I/R injury. Liver injury was assessed by measuring serum alanine aminotransferase (ALT) level, necrotic areas by liver histology, hepatocyte death, and local hepatic inflammatory responses. Signal pathways were explored in vivo and in vitro to identify possible mechanisms for TSLP in I/R injury. TSLP and TSLPR protein expression increased during liver I/R in vivo and following hepatocyte hypoxia/reoxygenation in vitro. Deletion of TSLPR or neutralization of TSLP with anti-TSLP antibody exacerbated liver injury in terms of serum ALT levels as well as necrotic areas in liver histology. Administration of exogenous recombinant mouse TSLP to WT mice significantly reduced liver damage compared with controls, but failed to prevent I/R injury in TSLPR-/- mice. TSLP induced autophagy in hepatocytes during liver I/R injury. Mechanistically, Akt was activated in WT mice during liver I/R injury. The opposite results were observed in TSLPR-/- mice. In addition, TSLP could directly induce Akt activation in hepatocytes independent of nonparenchymal cells in vitro. Furthermore, the Akt agonist, insulin-like growth factor-1 (IGF-1), prevented I/R injury in TSLPR-/- mice and an Akt inhibitor, LY294002, blocked the protective effects of TSLP in WT mice subjected to I/R. Our data indicate that TSLP protects against liver I/R injury via activation of the PI3K/Akt pathway. Through this pathway, TSLP induces autophagy in hepatocytes. Thus, TSLP is a potent inhibitor of stress-induced hepatocyte necrosis.
Keywords: Apoptosis; Hepatology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

