Context-dependent activation of SIRT3 is necessary for anchorage-independent survival and metastasis of ovarian cancer cells
- PMID: 31723239
- PMCID: PMC7036012
- DOI: 10.1038/s41388-019-1097-7
Context-dependent activation of SIRT3 is necessary for anchorage-independent survival and metastasis of ovarian cancer cells
Abstract
Tumor cells must alter their antioxidant capacity for maximal metastatic potential. Yet the antioxidant adaptations required for ovarian cancer transcoelomic metastasis, which is the passive dissemination of cells in the peritoneal cavity, remain largely unexplored. Somewhat contradicting the need for oxidant scavenging are previous observations that expression of SIRT3, a nutrient stress sensor and regulator of mitochondrial antioxidant defenses, is often suppressed in many primary tumors. We have discovered that this mitochondrial deacetylase is specifically upregulated in a context-dependent manner in cancer cells. SIRT3 activity and expression transiently increased following ovarian cancer cell detachment and in tumor cells derived from malignant ascites of high-grade serous adenocarcinoma patients. Mechanistically, SIRT3 prevents mitochondrial superoxide surges in detached cells by regulating the manganese superoxide dismutase (SOD2). This mitochondrial stress response is under dual regulation by SIRT3. SIRT3 rapidly increases SOD2 activity as an early adaptation to cellular detachment, which is followed by SIRT3-dependent increases in SOD2 mRNA during sustained anchorage-independence. In addition, SIRT3 inhibits glycolytic capacity in anchorage-independent cells thereby contributing to metabolic changes in response to detachment. While manipulation of SIRT3 expression has few deleterious effects on cancer cells in attached conditions, SIRT3 upregulation and SIRT3-mediated oxidant scavenging are required for anoikis resistance in vitro following matrix detachment, and both SIRT3 and SOD2 are necessary for colonization of the peritoneal cavity in vivo. Our results highlight the novel context-specific, pro-metastatic role of SIRT3 in ovarian cancer.
Conflict of interest statement
Conflict of Interest:
The authors declare no competing financial interests in relation to the work described.
Figures


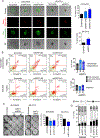
 ), ascites (
), ascites ( ), and peritoneal or omental lesions (
), and peritoneal or omental lesions ( ; Geo:GSE85296, Pearson correlation).
; Geo:GSE85296, Pearson correlation).


References
-
- Sehouli J, Senyuva F, Fotopoulou C, Neumann U, Denkert C, Werner L et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol 2009; 99: 424–427. - PubMed
-
- Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. The lancet oncology 2006; 7: 925–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

