Using Chemical Knowledge to Uncover New Biological Function: Discovery of the Cylindrocyclophane Biosynthetic Pathway
- PMID: 31723311
- PMCID: PMC6853628
- DOI: 10.1055/s-0033-1338879
Using Chemical Knowledge to Uncover New Biological Function: Discovery of the Cylindrocyclophane Biosynthetic Pathway
Abstract
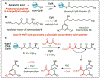
An understanding of organic chemistry can play a central role in uncovering enzymes with new biochemical functions. We have recently identified the enzymes involved in the biosynthesis of the cylindrocyclophanes, a structurally unique family of natural products, and found that this pathway employs a remarkable macrocyclization event that requires functionalization of an unactivated carbon atom. This work illustrates the potential of using chemically guided approaches for enzyme discovery.
Keywords: Biosynthesis; cylindrocyclophanes; enzymes; genomics; natural products.
Figures
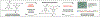

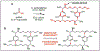

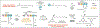


Similar articles
-
Cylindrocyclophane biosynthesis involves functionalization of an unactivated carbon center.J Am Chem Soc. 2012 Nov 14;134(45):18518-21. doi: 10.1021/ja308318p. Epub 2012 Nov 2. J Am Chem Soc. 2012. PMID: 23106426 Free PMC article.
-
Leveraging Microbial Genomes and Genomic Context for Chemical Discovery.Acc Chem Res. 2021 Jul 6;54(13):2788-2797. doi: 10.1021/acs.accounts.1c00100. Epub 2021 Jun 4. Acc Chem Res. 2021. PMID: 34087065 Free PMC article.
-
A new strategy for aromatic ring alkylation in cylindrocyclophane biosynthesis.Nat Chem Biol. 2017 Aug;13(8):916-921. doi: 10.1038/nchembio.2421. Epub 2017 Jun 26. Nat Chem Biol. 2017. PMID: 28671684
-
Biosynthesis of Lincosamide Antibiotics: Reactions Associated with Degradation and Detoxification Pathways Play a Constructive Role.Acc Chem Res. 2018 Jun 19;51(6):1496-1506. doi: 10.1021/acs.accounts.8b00135. Epub 2018 May 24. Acc Chem Res. 2018. PMID: 29792672 Review.
-
Linking Genes to Molecules in Eukaryotic Sources: An Endeavor to Expand Our Biosynthetic Repertoire.Molecules. 2020 Jan 31;25(3):625. doi: 10.3390/molecules25030625. Molecules. 2020. PMID: 32023950 Free PMC article. Review.
Cited by
-
Effects of Halide Ions on the Carbamidocyclophane Biosynthesis in Nostoc sp. CAVN2.Mar Drugs. 2016 Jan 20;14(1):21. doi: 10.3390/md14010021. Mar Drugs. 2016. PMID: 26805858 Free PMC article.
-
Chemistry, bioactivity and biosynthesis of cyanobacterial alkylresorcinols.Nat Prod Rep. 2019 Oct 16;36(10):1437-1461. doi: 10.1039/c8np00080h. Nat Prod Rep. 2019. PMID: 30702733 Free PMC article. Review.
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
Biosynthesis-assisted structural elucidation of the bartolosides, chlorinated aromatic glycolipids from cyanobacteria.Angew Chem Int Ed Engl. 2015 Sep 14;54(38):11063-7. doi: 10.1002/anie.201503186. Epub 2015 Jul 31. Angew Chem Int Ed Engl. 2015. PMID: 26235728 Free PMC article.
-
Combining Mass Spectrometric Metabolic Profiling with Genomic Analysis: A Powerful Approach for Discovering Natural Products from Cyanobacteria.J Nat Prod. 2015 Jul 24;78(7):1671-82. doi: 10.1021/acs.jnatprod.5b00301. Epub 2015 Jul 7. J Nat Prod. 2015. PMID: 26149623 Free PMC article.
References
-
- Koeller KM; Wong C-H Nature 2001, 409, 232. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
