Directed Collective Cell Migration Using Three-Dimensional Bioprinted Micropatterns on Thermoresponsive Surfaces for Myotube Formation
- PMID: 31723595
- PMCID: PMC6853623
- DOI: 10.1021/acsbiomaterials.8b01359
Directed Collective Cell Migration Using Three-Dimensional Bioprinted Micropatterns on Thermoresponsive Surfaces for Myotube Formation
Abstract
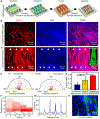
Directed collective cell migration governs cell orientation during tissue morphogenesis, wound healing, and tumor metastasis. Unfortunately, current methods for initiating collective cell migration, such as scratching, laser ablation, and stencils, either introduce uncontrolled cell-injury, involve multiple fabrication processes, or have utility limited to cells with strong cell-cell junctions. Using three-dimensional (3D) bioprinted gelatin methacryloyl (GelMA) micropatterns on temperature-responsive poly(N-isopropylacrylamide) (PNIPAm) coated interfaces, we demonstrate that directed injury-free collective cell migration could occur in parallel and perpendicular directions. After seeding cells, we created cell-free spaces between two 3D bioprinted GelMA micropatterns by lowering the temperature of PNIPAm interfaces to promote the cell detachment. Unlike conventional collective cell migration methods initiated by stencils, we observed well-organized cell migration in parallel and perpendicular to 3D bioprinted micropatterns as a function of the distance between 3D bioprinted micropatterns. We further established the utility of controlled collective cell migration for directed functional myotube formation using 3D bioprinted fingerprintlike micropatterns as well as iris musclelike concentric circular patterns. Our platform is unique for myoblast alignment and myotube formation because it does not require anisotropic guidance cues. Together, our findings establish how to achieve controlled collective cell migration, even at the macroscale, for tissue engineering and regeneration.
Keywords: 3D printing; directed collective cell migration; myotube orientation; temperature-responsive surface.
Figures
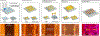





References
-
- Haeger A; Wolf K; Zegers MM; Friedl P Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015, 25 (9), 556–566. - PubMed
-
- Vedula SRK; Hirata H; Nai MH; Brugues A; Toyama Y; Trepat X; Lim CT; Ladoux B Epithelial bridges maintain tissue integrity during collective cell migration. Nat. Mater. 2014,13 (1), 87–96. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
