Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe
- PMID: 31723747
- PMCID: PMC6745952
- DOI: 10.1097/HS9.0000000000000018
Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe
Abstract
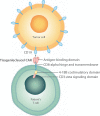
Chimeric antigen receptor (CAR)-T cell therapy is a new class of cellular immunotherapies that involves ex vivo genetic modification of T cells to incorporate an engineered CAR. After infusion into the patient, the CAR-expressing T cells recognize specific tumor targets and induce an immune response against them. The technology utilized is fundamentally different from previously available cancer treatments. Currently, most CAR-T cell therapies use autologous T cells. Tisagenlecleucel (formerly CTL019) is an anti-CD19 CAR-T cell therapy that was recently approved in the United States for the treatment of pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL). Tisagenlecleucel has shown robust in vivo expansion and long-term persistence, clinically meaningful durable response and remission rates, and overall survival benefit in pediatric and young adult patients with relapsed/refractory B-ALL and in relapsed/refractory diffuse large B-cell lymphoma. Common adverse events (AEs) include cytokine release syndrome, which may require hospitalization and admission to an intensive care unit, neurological toxicities, and B-cell aplasia. These AEs are manageable when treated by an appropriately trained team. Additional research is required to further develop AE management protocols. In this review, we describe regulatory requirements, clinical considerations, and site-level requirements for clinical study implementation of CAR-T cell therapy in Europe. We also provide a case study of the European experience from the first global clinical trial for tisagenlecleucel, which may serve as a useful starting point for investigators and clinicians looking to implement CAR-T cell therapy at their institutions.
Copyright © 2018 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures
References
-
- Maude S, Teachey D, Rheingold S, et al. Durable remissions after monotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children and young adults with relapsed/refractory ALL [Abstract S497]. Haematologica 2016; 101 (suppl 1):183–184.
-
- Maude SL, Pulsipher MA, Boyer MW, et al. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis [Abstract 2801]. Blood 2016; 128:2801.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources