The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics
- PMID: 31724044
- PMCID: PMC6853763
- DOI: 10.1093/cid/ciz825
The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics
Abstract
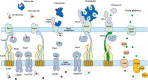
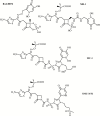
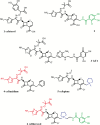
Iron is an essential nutrient for bacterial growth, replication, and metabolism. Humans store iron bound to various proteins such as hemoglobin, haptoglobin, transferrin, ferritin, and lactoferrin, limiting the availability of free iron for pathogenic bacteria. However, bacteria have developed various mechanisms to sequester or scavenge iron from the host environment. Iron can be taken up by means of active transport systems that consist of bacterial small molecule siderophores, outer membrane siderophore receptors, the TonB-ExbBD energy-transducing proteins coupling the outer and the inner membranes, and inner membrane transporters. Some bacteria also express outer membrane receptors for iron-binding proteins of the host and extract iron directly from these for uptake. Ultimately, iron is acquired and transported into the bacterial cytoplasm. The siderophores are small molecules produced and released by nearly all bacterial species and are classified according to the chemical nature of their iron-chelating group (ie, catechol, hydroxamate, α-hydroxyl-carboxylate, or mixed types). Siderophore-conjugated antibiotics that exploit such iron-transport systems are under development for the treatment of infections caused by gram-negative bacteria. Despite demonstrating high in vitro potency against pathogenic multidrug-resistant bacteria, further development of several candidates had stopped due to apparent adaptive resistance during exposure, lack of consistent in vivo efficacy, or emergence of side effects in the host. However, cefiderocol, with an optimized structure, has advanced and has been investigated in phase 1 to 3 clinical trials. This article discusses the mechanisms implicated in iron uptake and the challenges associated with the design and utilization of siderophore-mimicking antibiotics.
Keywords: iron transport; monobactams; siderophore; siderophore-antibiotic conjugate; β-lactams.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Tansarli GS, Karageorgopoulos DE, Kapaskelis A, Gkegkes I, Falagas ME. Iron deficiency and susceptibility to infections: evaluation of the clinical evidence. Eur J Clin Microbiol Infect Dis 2013; 32:1253–8. - PubMed
-
- Kontoghiorghes GJ, Kolnagou A, Skiada A, Petrikkos G. The role of iron and chelators on infections in iron overload and non iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin 2010; 34:227–39. - PubMed
-
- Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron— a metal at the host-pathogen interface. Cell Microbiol 2010; 12:1691–702. - PubMed
-
- Brock JH. Benefits and dangers of iron during infection. Curr Opin Clin Nutr Metab Care 1999; 2:507–10. - PubMed
-
- Kasvosve I, Delanghe J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med 2002; 40:1014–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

