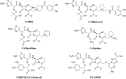
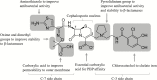
Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin
- PMID: 31724047
- PMCID: PMC6853759
- DOI: 10.1093/cid/ciz826
Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin
Abstract
The emergence of antimicrobial resistance is a significant public health issue worldwide, particularly for healthcare-associated infections caused by carbapenem-resistant gram-negative pathogens. Cefiderocol is a novel siderophore cephalosporin targeting gram-negative bacteria, including strains with carbapenem resistance. The structural characteristics of cefiderocol show similarity to both ceftazidime and cefepime, which enable cefiderocol to withstand hydrolysis by β-lactamases. The unique chemical component is the addition of a catechol moiety on the C-3 side chain, which chelates iron and mimics naturally occurring siderophore molecules. Following the chelation of iron, cefiderocol is actively transported across the outer membrane of the bacterial cell to the periplasmic space via specialized iron transporter channels. Furthermore, cefiderocol has demonstrated structural stability against hydrolysis by both serine- and metallo-β-lactamases, including clinically relevant carbapenemases such as Klebsiella pneumoniae carbapenemase, oxacillin carbapenemase-48, and New Delhi metallo-β-lactamase. Cefiderocol has demonstrated promising in vitro antibacterial and bactericidal activity, which correlates with its in vivo efficacy in several animal models. This article reviews the discovery and chemistry of cefiderocol, as well as some of the key microbiological and in vivo findings on cefiderocol from recently conducted investigations.
Keywords: carbapenemase; cephalosporin; gram-negative bacteria; penicillin-binding protein 3; siderophore.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Ghosh A, Ghosh M, Niu C, Malouin F, Moellmann U, Miller MJ. Iron transport-mediated drug delivery using mixed-ligand siderophore-beta-lactam conjugates. Chem Biol 1996; 3:1011–9. - PubMed
-
- Miyakawa S, Noto T, Okazaki H. Mechanisms of resistance in Escherichia coli to the sideromycin antibiotic no. 216: isolation and characterization of the resistant mutants. Microbiol Immunol 1982; 26:885–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous