Pathogen-focused Clinical Development to Address Unmet Medical Need: Cefiderocol Targeting Carbapenem Resistance
- PMID: 31724048
- PMCID: PMC6853756
- DOI: 10.1093/cid/ciz829
Pathogen-focused Clinical Development to Address Unmet Medical Need: Cefiderocol Targeting Carbapenem Resistance
Abstract
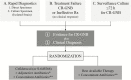
Historically, the regulatory requirements of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for developing new antibiotics have not addressed pathogen-focused indications for drug approval. The design of the necessary randomized controlled trials traditionally involves the enrollment of patients with site-specific infections caused by susceptible as well as resistant pathogens. Cefiderocol has undergone a streamlined clinical development program to address serious carbapenem-resistant infections. The regulatory approach, and the pivotal clinical trials, differed between the FDA and EMA. In the United States, the APEKS-cUTI (Acinetobacter, Pseudomonas, Escherichia coli, Klebsiella, Stenotrophomonas-complicated urinary tract infection) study was conducted to provide the basis for FDA approval of a site-specific cUTI indication. The EMA, however, preferred the CREDIBLE-CR (A MultiCenter, RandomizED, Open-label ClInical Study of S-649266 or Best AvailabLE Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-negative Pathogens) study, in which patients with nosocomial pneumonia, bloodstream infections, or cUTIs were enrolled if they had a carbapenem-resistant pathogen. The resulting European label will be pathogen focused rather than infection site specific (ie, treatment of gram-negative infection in patients with limited treatment options). The implications and limitations of these different regulatory processes are discussed.
Keywords: carbapenem resistance; cefiderocol; nonfermenters; pathogen-focused drug development; regulatory drug approval.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe—annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), 2017 Available at: https://ecdc.europa.eu/sites/portal/files/documents/EARS-Net-report-2017.... Accessed 3 March 2019.
-
- World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. Available at: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET.... Accessed 1 February 2019.
-
- Aoki T, Yoshizawa H, Yamawaki K, et al. . Cefiderocol (S-649266), a new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: structure activity relationship. Eur J Med Chem 2018; 155:847–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

