Metarhizium brunneum infection dynamics differ at the cuticle interface of susceptible and tolerant morphs of Galleria mellonella
- PMID: 31724467
- PMCID: PMC8647853
- DOI: 10.1080/21505594.2019.1693230
Metarhizium brunneum infection dynamics differ at the cuticle interface of susceptible and tolerant morphs of Galleria mellonella
Abstract
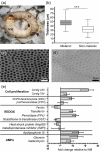
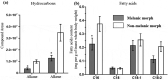
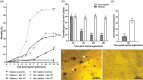
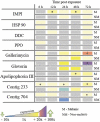
In order for entomopathogenic fungi to colonize an insect host, they must first attach to, and penetrate, the cuticle layers of the integument. Herein, we explored the interactions between the fungal pathogen Metarhizium brunneum ARSEF 4556 and two immunologically distinct morphs, melanic (M) and non-melanic (NM), of the greater wax moth Galleria mellonella. We first interrogated the cuticular compositions of both insect morphs to reveal substantial differences in their physiochemical properties. Enhanced melanin accumulation, fewer hydrocarbons, and higher L-dihydroxyphenylalanine (DOPA) decarboxylase activity were evident in the cuticle of the M larvae. This "hostile" terrain proved challenging for M. brunneum - reflected in poor conidial attachment and germination, and elevated expression of stress-associated genes (e.g., Hsp30, Hsp70). Lack of adherence to the cuticle impacted negatively on the speed of kill and overall host mortality; a dose of 107 conidia killed ~30% of M larvae over a 12-day period, whereas a 100-fold lower dose (105 conidia) achieved a similar result for NM larvae. Candidate gene expression patterns between the insect morphs indicated that M larvae are primed to "switch-on" immunity-associated genes (e.g., phenoloxidase) within 6-12 h of conidia exposure and can sustain a "defense" response. Critically, M. brunneum responds to the distinct physiochemical cues of both hosts and adjusts the expression of pathogenicity-related genes accordingly (e.g., Pr2, Mad1, Mad2). We reveal previously uncharacterized mechanisms of attack and defence in fungal-insect antibiosis.
Keywords: Galleria mellonella; Innate immunity; cuticle; entomopathogenic fungi; host-pathogen interactions; melanization.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
-
- Liao C, Upadhyay A, Liang J, et al. 3, 4-Dihydroxyphenylacetaldehyde synthase and cuticle formation in insects. Dev Comp Immunol. 2018;83:pp.44–50. - PubMed
-
- Vincent JF, Wegst UG.. Design and mechanical properties of insect cuticle. Arthropod Struct Dev. 2004;33(3):pp.187–199. - PubMed
-
- Butt TM, Coates CJ, Dubovskiy IM, et al. Entomopathogenic fungi: new insights into host–pathogen interactions. In: Advances in genetics. Vol. 94. Academic Press; 2016. p. 307–364. - PubMed
-
- de Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43(3):237–256.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
