Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164
- PMID: 31725351
- PMCID: PMC7031958
- DOI: 10.1200/JCO.19.02107
Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164
Abstract
Purpose: KEYNOTE-164 (NCT02460198) evaluated the antitumor activity of pembrolizumab in previously treated, metastatic, microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) colorectal cancer (CRC).
Methods: This phase II open-label study involved 128 centers worldwide. Eligible patients were age ≥ 18 years and had metastatic MSI-H/dMMR CRC treated with ≥ 2 prior lines of standard therapy, including fluoropyrimidine, oxaliplatin, and irinotecan with or without anti-vascular endothelial growth factor/epidermal growth factor receptor monoclonal antibody (cohort A) or ≥ 1 prior line of therapy (cohort B). MSI-H/dMMR status was assessed locally. Patients received pembrolizumab 200 mg every 3 weeks for up to 2 years until progression, unacceptable toxicity, or withdrawal. The primary end point was objective response rate by RECIST version 1.1 by independent central review. Secondary end points were duration of response, progression-free survival (PFS), overall survival, safety, and tolerability.
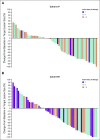
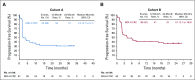
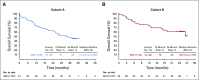
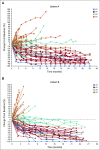
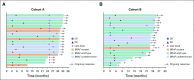
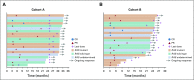
Results: A total of 124 patients with MSI-H/dMMR CRC (61 in cohort A, 63 in cohort B) enrolled. At data cutoff, median follow-up was 31.3 months (range, 0.2-35.6 months) for cohort A and 24.2 months (range, 0.1-27.1 months) for cohort B. Objective response rate was 33% (95% CI, 21% to 46%) and 33% (95% CI, 22% to 46%), respectively, with median duration of response not reached in either cohort. Median PFS was 2.3 months (95% CI, 2.1 to 8.1 months) and 4.1 months (95% CI, 2.1 to 18.9 months). Median overall survival was 31.4 months (95% CI, 21.4 months to not reached) and not reached (95% CI, 19.2 months to not reached). Treatment-related grade 3-4 adverse events occurred in 10 patients (16%) in cohort A and 8 (13%) in cohort B, with the most common occurring in ≥ 2 patients being pancreatitis, fatigue, increased alanine aminotransferase, and increased lipase (2 patients each; 3%) in cohort A.
Conclusion: Pembrolizumab is effective with a manageable safety profile in patients with MSI-H/dMMR CRC.
Figures
Comment in
-
MSI-H: a truly agnostic biomarker?Nat Rev Clin Oncol. 2020 Feb;17(2):68. doi: 10.1038/s41571-019-0310-5. Nat Rev Clin Oncol. 2020. PMID: 31831852 No abstract available.
References
-
- International Agency for Research on Cancer: Cancer Fact Sheet, March 2019. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sh....
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

