Frequency cluster formation and slow oscillations in neural populations with plasticity
- PMID: 31725782
- PMCID: PMC6855470
- DOI: 10.1371/journal.pone.0225094
Frequency cluster formation and slow oscillations in neural populations with plasticity
Abstract
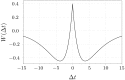
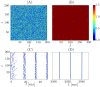
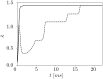
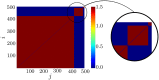
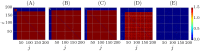
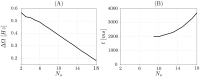
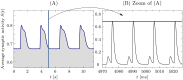
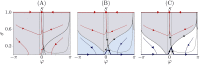
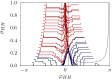
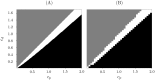
We report the phenomenon of frequency clustering in a network of Hodgkin-Huxley neurons with spike timing-dependent plasticity. The clustering leads to a splitting of a neural population into a few groups synchronized at different frequencies. In this regime, the amplitude of the mean field undergoes low-frequency modulations, which may contribute to the mechanism of the emergence of slow oscillations of neural activity observed in spectral power of local field potentials or electroencephalographic signals at high frequencies. In addition to numerical simulations of such multi-clusters, we investigate the mechanisms of the observed phenomena using the simplest case of two clusters. In particular, we propose a phenomenological model which describes the dynamics of two clusters taking into account the adaptation of coupling weights. We also determine the set of plasticity functions (update rules), which lead to multi-clustering.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

