Gut mycobiota under scrutiny: fungal symbionts or environmental transients?
- PMID: 31726316
- PMCID: PMC6908457
- DOI: 10.1016/j.mib.2019.09.010
Gut mycobiota under scrutiny: fungal symbionts or environmental transients?
Abstract
The human gastrointestinal tract is home to a thriving community of microbes including the fungal 'mycobiota'. Although sequencing methodology has enumerated diverse fungal genera within this niche, discerning persistent symbiotic residents from contaminants and purely environmental transients remains a challenge. Recent advances in culturomics and sequencing employing metagenomics, metatranscriptomics and longitudinal studies have begun to reveal a human symbiont 'core mycobiome' that may contribute to human health and disease. Trans-kingdom interactions between the bacterial microbiota and evolution within the niche have defined C. albicans as a true symbiont, setting a bar for defining other fungi. Additionally, elegant investigations of mammalian antifungal immunity have examined mononuclear phagocytes, neutrophils, antigen-specific recognition by T cells and other mechanisms important for local and systemic effects on the host, providing further evidence supporting gut persistence. In this review we discuss current research aimed at investigating the symbiotic mycobiota and propose four criteria aiding in the differentiation of fungal symbionts from environmental transients.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
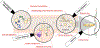
References
-
- Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, Ramer-Tait AE, Iliev ID, Response to Fungal Dysbiosis by Gut-Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease, Cell Host Microbe. 24 (2018) 847–856.e4. doi:10.1016/j.chom.2018.11.003. - DOI - PMC - PubMed
-
*The study reports an expansion of Th2 cells in the lung upon gut fungal dysbiosis. The observed systemic responses to fungi in the gut are mediated by gut-resident CX3CR1+ mononuclear phagocytes.
-
- Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan KG, Yurieva M, Liao W, Poidinger M, Zolezzi F, Rancati G, Pavelka N, Experimental evolution of a fungal pathogen into a gut symbiont, Science. 362 (2018) 589–595. doi:10.1126/science.aat0537. - DOI - PubMed
-
*Authors were able to evolve C. albicans through serial gastrointestinal passage experiments into hyper-competitive strains often resulting from Flo8 transcriptional factor mutations. Interesting, a few strains bestowed protective benefits to the host from a range of pathogens including A. fumigatus, P. aeruginosa, S. aureus, and C. albicans, in a T and B cell independent manner.
-
- Liang S-H, Anderson MZ, Hirakawa MP, Wang JM, Frazer C, Alaalm LM, Thomson GJ, Ene IV, Bennett RJ, Hemizygosity Enables a Mutational Transition Governing Fungal Virulence and Commensalism, Cell Host Microbe. 25 (2019) 418–431.e6. doi:10.1016/j.chom.2019.01.005. - DOI - PMC - PubMed
-
** Authors investigated phenotypic switching in C. albicans and revealed that the genetic basis for metabolic states is dependent on zygosity of the transcription factor EFG1 in combination with the mating locus a1/a2. Oral C. albicans strains containing hemizygous EFG1 were isolated from patients and this genetic state was found to confer increased fitness in the gastrointestinal tract and increased mortality in a mouse model of systemic candidiasis.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

