Combinatory Multifactor Treatment Effects on Primary Nanofiber Oligodendrocyte Cultures
- PMID: 31726669
- PMCID: PMC6912369
- DOI: 10.3390/cells8111422
Combinatory Multifactor Treatment Effects on Primary Nanofiber Oligodendrocyte Cultures
Abstract
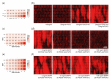
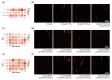
Multiple sclerosis (MS) is a chronic inflammatory demyelinating and neurodegenerative disease of the central nervous system. Neurological deficits are attributed to inflammatory demyelination, which compromises axonal function and survival. These are mitigated in experimental models by rapid and often complete remyelination of affected axons, but in MS this endogenous repair mechanism frequently fails, leaving axons increasingly vulnerable to the detrimental effects of inflammatory and metabolic stress. Understanding the molecular basis of remyelination and remyelination failure is essential to develop improved therapies for this devastating disease. However, recent studies suggest that this is not due to a single dominant mechanism, but rather represents the biological outcome of multiple changes in the lesion microenvironment that combine to disrupt oligodendrocyte differentiation. This identifies a pressing need to develop technical platforms to investigate combinatory and/or synergistic effects of factors differentially expressed in MS lesions on oligodendrocyte proliferation and differentiation. Here we describe protocols using primary oligodendrocyte cultures from Bl6 mice on 384-well nanofiber plates to model changes affecting oligodendrogenesis and differentiation in the complex signaling environment associated with multiple sclerosis lesions. Using platelet-derived growth factor (PDGF-AA), fibroblast growth factor 2 (FGF2), bone morphogenetic protein 2 (BMP2) and bone morphogenetic protein 4 (BMP4) as representative targets, we demonstrate that we can assess their combinatory effects across a wide range of concentrations in a single experiment. This in vitro model is ideal for assessing the combinatory effects of changes in availability of multiple factors, thus more closely modelling the situation in vivo and furthering high-throughput screening possibilities.
Keywords: multiple sclerosis; myelin; nanofibers; oligodendrocyte; remyelination; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter.J Neurosci. 2002 Oct 1;22(19):8574-85. doi: 10.1523/JNEUROSCI.22-19-08574.2002. J Neurosci. 2002. PMID: 12351731 Free PMC article.
-
Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination.PLoS One. 2017 Dec 18;12(12):e0189380. doi: 10.1371/journal.pone.0189380. eCollection 2017. PLoS One. 2017. PMID: 29253893 Free PMC article.
-
Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo.Nature. 2015 Jun 11;522(7555):216-20. doi: 10.1038/nature14335. Epub 2015 Apr 20. Nature. 2015. PMID: 25896324 Free PMC article.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
Remyelinating strategies in multiple sclerosis.Expert Rev Neurother. 2014 Nov;14(11):1315-34. doi: 10.1586/14737175.2014.969241. Expert Rev Neurother. 2014. PMID: 25331418 Review.
Cited by
-
Oligodendrocyte Physiology and Pathology Function.Cells. 2020 Sep 11;9(9):2078. doi: 10.3390/cells9092078. Cells. 2020. PMID: 32932835 Free PMC article.
-
Nanomaterial payload delivery to central nervous system glia for neural protection and repair.Front Cell Neurosci. 2023 Oct 24;17:1266019. doi: 10.3389/fncel.2023.1266019. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37941607 Free PMC article. Review.
-
An in vivo accelerated developmental myelination model for testing promyelinating therapeutics.BMC Neurosci. 2022 May 25;23(1):30. doi: 10.1186/s12868-022-00714-y. BMC Neurosci. 2022. PMID: 35614392 Free PMC article.
-
An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events.Antioxidants (Basel). 2020 Mar 5;9(3):216. doi: 10.3390/antiox9030216. Antioxidants (Basel). 2020. PMID: 32150935 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

