Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial
- PMID: 31727113
- PMCID: PMC6854749
- DOI: 10.1186/s13058-019-1215-z
Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial
Abstract
Background: In this prospective phase 2 trial, we assessed the efficacy of trastuzumab-emtansine (T-DM1) in HER2-negative metastatic breast cancer (MBC) patients with HER2-positive CTC.
Methods: Main inclusion criteria for screening were as follows: women with HER2-negative MBC treated with ≥ 2 prior lines of chemotherapy and measurable disease. CTC with a HER2/CEP17 ratio of ≥ 2.2 by fluorescent in situ hybridization (CellSearch) were considered to be HER2-amplified (HER2amp). Patients with ≥ 1 HER2amp CTC were eligible for the treatment phase (T-DM1 monotherapy). The primary endpoint was the overall response rate.
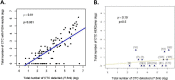
Results: In 154 screened patients, ≥ 1 and ≥ 5 CTC/7.5 ml of blood were detected in N = 118 (78.7%) and N = 86 (57.3%) patients, respectively. ≥1 HER2amp CTC was found in 14 patients (9.1% of patients with ≥ 1 CTC/7.5 ml). Among 11 patients treated with T-DM1, one achieved a confirmed partial response. Four patients had a stable disease as best response. Median PFS was 4.8 months while median OS was 9.5 months.
Conclusions: CTC with HER2 amplification can be detected in a limited subset of HER2-negative MBC patients. Treatment with T-DM1 achieved a partial response in only one patient.
Trial registration: NCT01975142, Registered 03 November 2013.
Keywords: Circulating tumor cells; HER2; Liquid biopsy; Metastatic breast cancer; Trastuzumab-emtansine.
Conflict of interest statement
WJ: honoraria and travel grants from Roche. PC: research support from Roche. FCB and JYP: research support from Janssen Diagnostics, research support, honoraria, and travel grants from Roche. The other authors declare that they have no competing interests.
Figures


References
-
- Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. - DOI - PubMed
-
- Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JMS, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. - DOI - PubMed
-
- Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, Adamoli L, Munzone E, Sciandivasci A, De Vita F, Goldhirsch A, Nolè F. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50:277–289. doi: 10.1016/j.ejca.2013.10.004. - DOI - PubMed
-
- Comte A, Sigal-Zafrani B, Belin L, Bièche I, Callens C, Diéras V, Bidard F-C, Mariani O, Servois V, Szwarc D, Vincent-Salomon A, Brain ECG, et al. Abstract P2-05-06: Clinical utility of systematic biopsy of first metastatic event in breast cancer: Results from a prospective multicenter trial. Cancer Res. 2016;76:P2–05–P2–06.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

