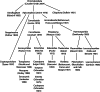
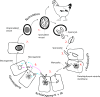
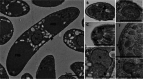
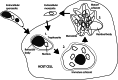
Life cycle stages, specific organelles and invasion mechanisms of Eimeria species
- PMID: 31727204
- PMCID: PMC10317661
- DOI: 10.1017/S0031182019001562
Life cycle stages, specific organelles and invasion mechanisms of Eimeria species
Abstract
Apicomplexans, including species of Eimeria, pose a real threat to the health and wellbeing of animals and humans. Eimeria parasites do not infect humans but cause an important economic impact on livestock, in particular on the poultry industry. Despite its high prevalence and financial costs, little is known about the cell biology of these 'cosmopolitan' parasites found all over the world. In this review, we discuss different aspects of the life cycle and stages of Eimeria species, focusing on cellular structures and organelles typical of the coccidian family as well as genus-specific features, complementing some 'unknowns' with what is described in the closely related coccidian Toxoplasma gondii.
Keywords: Cell biology; Eimeria; coccidia; invasion; life cycle stages; secretory organelles.
Figures


References
-
- Adl SM, Leander BS, Simpson AG, Archibald JM, Anderson OR, Bass D, Bowser SS, Brugerolle G, Farmer MA, Karpov S, Kolisko M, Lane CE, Lodge DJ, Mann DG, Meisterfeld R, Mendoza L, Moestrup O, Mozley-Standridge SE, Smirnov AV and Spiegel F (2007) Diversity, nomenclature, and taxonomy of protists. Systematic Biology 56, 684–689. - PubMed
-
- Augustine PC (2001) Cell: sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria. International Journal for Parasitology 31, 1–8. - PubMed
-
- Behrendt JH, Clauss W, Zahner H and Hermosilla C (2004) Alternative mechanism of Eimeria bovis sporozoites to invade cells in vitro by breaching the plasma membrane. Journal of Parasitology 90, 1163–1165. - PubMed
-
- Belli SI, Smith NC and Ferguson DJ (2006) The coccidian oocyst: a tough nut to crack!. Trends in Parasitology 22, 416–423. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

