TAME trial: a multi-arm phase II randomised trial of four novel interventions for malnutrition enteropathy in Zambia and Zimbabwe - a study protocol
- PMID: 31727642
- PMCID: PMC6887014
- DOI: 10.1136/bmjopen-2018-027548
TAME trial: a multi-arm phase II randomised trial of four novel interventions for malnutrition enteropathy in Zambia and Zimbabwe - a study protocol
Abstract
Introduction: Severe acute malnutrition (SAM) in children in many countries still carries unacceptably high mortality, especially when complicated by secondary infection or metabolic derangements. New therapies are urgently needed and we have identified mucosal healing in the intestine as a potential target for novel treatment approaches.
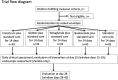
Methods and analysis: The TAME trial (Therapeutic Approaches for Malnutrition Enteropathy) will evaluate four novel treatments in an efficient multi-arm single-blind phase II design. In three hospitals in Zambia and Zimbabwe, 225 children with SAM will be randomised to one of these treatments or to standard care, once their inpatient treatment has reached the point of transition from stabilisation to increased nutritional intake. The four interventions are budesonide, bovine colostrum or N-acetyl glucosamine given orally or via nasogastric tube, or teduglutide given by subcutaneous injection. The primary endpoint will be a composite score of faecal inflammatory markers, and a range of secondary endpoints include clinical and laboratory endpoints. Treatments will be given daily for 14 days, and evaluation of the major endpoints will be at 14 to 18 days, with a final clinical evaluation at 28 days. In a subset of children in Zambia, endoscopic biopsies will be used to evaluate the effect of interventions in detail.
Ethics and dissemination: The study has been approved by the University of Zambia Biomedical Research Ethics Committee (006-09-17, dated 9th July, 2018), and the Joint Research Ethics Committee of the University of Zimbabwe (24th July, 2019). Caregivers will provide written informed consent for each participant. Findings will be disseminated through peer-reviewed journals, conference presentations and to caregivers at face-to-face meetings.
Trial registration number: NCT03716115; Pre-results.
Keywords: clinical trials; nutrition & dietetics; paediatric gastroenterology; tropical medicine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- ICF Zambia demographic and health survey 2013-14; Zimbabwe demographic and health survey. Rockville, Maryland, USA: Central Statistical Office, Ministry of Health, and ICF International, 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical