Excitatory-Inhibitory Synaptic Coupling in Avian Nucleus Magnocellularis
- PMID: 31727796
- PMCID: PMC6961991
- DOI: 10.1523/JNEUROSCI.1124-19.2019
Excitatory-Inhibitory Synaptic Coupling in Avian Nucleus Magnocellularis
Abstract
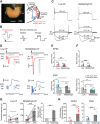
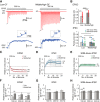
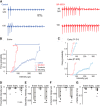
The activity of neurons is determined by the balance between their excitatory and inhibitory synaptic inputs. Neurons in the avian nucleus magnocellularis (NM) integrate monosynaptic excitatory and polysynaptic inhibitory inputs from the auditory nerve, and transmit phase-locked output to higher auditory centers. The excitatory input is graded tonotopically, such that neurons tuned to higher frequency receive fewer, but larger, axon terminals. However, it remains unknown how the balance between excitatory and inhibitory inputs is determined in NM. We here examined synaptic and spike responses of NM neurons during stimulation of the auditory nerve in thick brain slices of chicken of both sexes, and found that the excitatory-inhibitory balance varied according to tonotopic region, ensuring reliable spike output across frequencies. Auditory nerve stimulation elicited IPSCs in NM neurons regardless of tonotopic region, but the dependence of IPSCs on intensity varied in a systematic way. In neurons tuned to low frequency, IPSCs appeared and increased in parallel with EPSCs with elevation of intensity, which expanded dynamic range by preventing saturation of spike generation. On the other hand, in neurons tuned to higher frequency, IPSCs were smaller than EPSCs and had higher thresholds for activation, thus facilitating high-fidelity transmission. Computer simulation confirmed that these differences in inhibitory input were optimally matched to the patterns of excitatory input, and enabled appropriate level of neuronal output for wide intensity and frequency ranges of sound in the auditory system.SIGNIFICANCE STATEMENT Neurons in nucleus magnocellularis encode timing information of sound across wide intensity ranges by integrating excitatory and inhibitory synaptic inputs from the auditory nerve, but underlying synaptic mechanisms of this integration are not fully understood. We here show that the excitatory-inhibitory relationship was expressed differentially at each tonotopic region; the relationship was linear in neurons tuned to low-frequency, expanding dynamic range by preventing saturation of spike generation; by contrast inhibitory input remained much smaller than excitatory input in neurons tuned to higher frequency, thus ensuring high-fidelity transmission. The tonotopic regulation of excitatory and inhibitory input optimized the output across frequencies and intensities, playing a fundamental role in the timing coding pathway in the auditory system.
Keywords: GABA; auditory; brainstem; cochlear nucleus; inhibition; tonotopy.
Copyright © 2020 the authors.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
