Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase
- PMID: 31728173
- PMCID: PMC6839564
- DOI: 10.3762/bjoc.15.252
Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase
Abstract
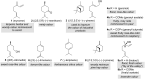
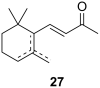
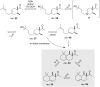
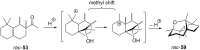
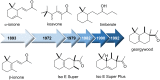
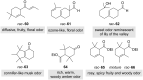
The history of fragrances is closely associated with the chemistry of terpenes and terpenoids. For thousands of years mankind mainly used plant extracts to collect ingredients for the creation of perfumes. Many of these extracts contain complex mixtures of terpenes, that show distinct olfactoric properties as pure compounds. When organic synthesis appeared on the scene, the portfolio of new scents increased either in order to substitute natural fragrances without change of olfactoric properties or to broaden the scope of scents. This short review describes the story of the most successful synthetic fragrance ever which is called Iso E Super® as it is an ingredient in a large number of perfumes with varying percentages and is the first example being used as a pure fragrance. Structurally, it is related to natural terpenes like many other synthetic fragrances. And indeed, the story began with a classic in the field of fragrances, the natural product ionone.
Keywords: asymmetric synthesis; fragrances; odorants; sandalwood; scents; terpenes; terpenoids.
Copyright © 2019, Stepanyuk and Kirschning; licensee Beilstein-Institut.
Figures


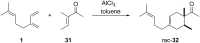

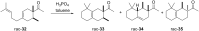





References
-
- Morris E T. Scents of Time: Perfume from Ancient Egypt to the 21st Century. Prestel; 1999.
-
- [Jul 8;2019 ];Fougere Royale - Houbigant Parfum Paris - Men’s Fragrances. Available from: https://www.houbigant-parfum.com/eu_en/fougere-royale.html/
-
- Sawamura M, Onishi Y, Ikemoto J, Tu N T M, Phi N T L. Flavour Fragrance J. 2006;21:609–615. doi: 10.1002/ffj.1604. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
