Simultaneous Production of Psilocybin and a Cocktail of β-Carboline Monoamine Oxidase Inhibitors in "Magic" Mushrooms
- PMID: 31729089
- PMCID: PMC7003923
- DOI: 10.1002/chem.201904363
Simultaneous Production of Psilocybin and a Cocktail of β-Carboline Monoamine Oxidase Inhibitors in "Magic" Mushrooms
Abstract
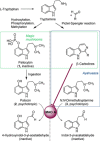
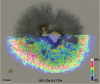
The psychotropic effects of Psilocybe "magic" mushrooms are caused by the l-tryptophan-derived alkaloid psilocybin. Despite their significance, the secondary metabolome of these fungi is poorly understood in general. Our analysis of four Psilocybe species identified harmane, harmine, and a range of other l-tryptophan-derived β-carbolines as their natural products, which was confirmed by 1D and 2D NMR spectroscopy. Stable-isotope labeling with 13 C11 -l-tryptophan verified the β-carbolines as biosynthetic products of these fungi. In addition, MALDI-MS imaging showed that β-carbolines accumulate toward the hyphal apices. As potent inhibitors of monoamine oxidases, β-carbolines are neuroactive compounds and interfere with psilocybin degradation. Therefore, our findings represent an unprecedented scenario of natural product pathways that diverge from the same building block and produce dissimilar compounds, yet contribute directly or indirectly to the same pharmacological effects.
Keywords: alkaloids; ayahuasca; beta-carboline; natural products; psilocybin.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schultes R. E., Amer. Anthropol. 1940, 42, 429–443.
-
- Fricke J., Lenz C., Wick J., Blei F., Hoffmeister D., Chem. Eur. J. 2019, 25, 897–903. - PubMed
-
- None
-
- Hofmann A., Heim R., Brack A., Kobel H., Experientia 1958, 14, 107–109; - PubMed
-
- Hofmann A., Heim R., Brack A., Kobel H., Frey A., Ott H., Petrzilka T., Troxler F., Helv. Chim. Acta 1959, 42, 1557–1572.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

