Virus-borne mini-CRISPR arrays are involved in interviral conflicts
- PMID: 31729390
- PMCID: PMC6858448
- DOI: 10.1038/s41467-019-13205-2
Virus-borne mini-CRISPR arrays are involved in interviral conflicts
Abstract
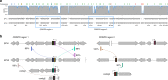
CRISPR-Cas immunity is at the forefront of antivirus defense in bacteria and archaea and specifically targets viruses carrying protospacers matching the spacers catalogued in the CRISPR arrays. Here, we perform deep sequencing of the CRISPRome-all spacers contained in a microbiome-associated with hyperthermophilic archaea of the order Sulfolobales recovered directly from an environmental sample and from enrichment cultures established in the laboratory. The 25 million CRISPR spacers sequenced from a single sampling site dwarf the diversity of spacers from all available Sulfolobales isolates and display complex temporal dynamics. Comparison of closely related virus strains shows that CRISPR targeting drives virus genome evolution. Furthermore, we show that some archaeal viruses carry mini-CRISPR arrays with 1-2 spacers and preceded by leader sequences but devoid of cas genes. Closely related viruses present in the same population carry spacers against each other. Targeting by these virus-borne spacers represents a distinct mechanism of heterotypic superinfection exclusion and appears to promote archaeal virus speciation.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

