Oxygen-catalysed sequential singlet fission
- PMID: 31729391
- PMCID: PMC6858316
- DOI: 10.1038/s41467-019-13202-5
Oxygen-catalysed sequential singlet fission
Abstract
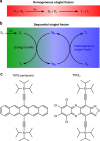
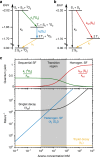
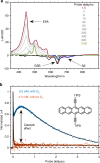
Singlet fission is the photoinduced conversion of a singlet exciton into two triplet states of half-energy. This multiplication mechanism has been successfully applied to improve the efficiency of single-junction solar cells in the visible spectral range. Here we show that singlet fission may also occur via a sequential mechanism, where the two triplet states are generated consecutively by exploiting oxygen as a catalyst. This sequential formation of carriers is demonstrated for two acene-like molecules in solution. First, energy transfer from the excited acene to triplet oxygen yields one triplet acene and singlet oxygen. In the second stage, singlet oxygen combines with a ground-state acene to complete singlet fission. This yields a second triplet molecule. The sequential mechanism accounts for approximately 40% of the triplet quantum yield in the studied molecules; this process occurs in dilute solutions and under atmospheric conditions, where the single-step SF mechanism is inactive.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kabir E, Kumar P, Kumar S, Adelodun AA, Kim K-H. Solar energy: potential and future prospects. Renew. Sust. Ener. Rev. 2018;82:894–900. doi: 10.1016/j.rser.2017.09.094. - DOI
Publication types
LinkOut - more resources
Full Text Sources

