A review of the NG17 recommendations for the use of basal insulin in type 1 diabetes
- PMID: 31729775
- PMCID: PMC7004078
- DOI: 10.1111/dme.14180
A review of the NG17 recommendations for the use of basal insulin in type 1 diabetes
Abstract
Aims: To revisit the data analysis used to inform National Institute of Health and Care Excellence (NICE) NG17 guidance for initiating basal insulin in adults with type 1 diabetes mellitus (diabetes).
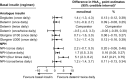
Methods: We replicated the data, methodology and analysis used by NICE diabetes in the NG17 network meta-analysis (NMA). We expanded this data cohort to a more contemporary data set (extended 2017 NMA) and restricted the studies included to improve the robustness of the data set (restricted 2017 NMA) and in a post hoc analysis, changed the index comparator from neutral protamine Hagedorn (NPH) insulin twice daily to insulin detemir twice daily.
Results: The absolute changes in HbA1c were similar to those reported in the NG17. However, all 95% credible intervals for change in HbA1c point estimates crossed the line of null effect, except for detemir twice daily (in the NICE and extended 2017 NMAs) and NPH four times daily. In the detemir twice-daily centred post hoc analysis, the 95% credible intervals for change in HbA1c crossed the line of null effect for all basal therapies, except NPH.
Conclusions: In NG17, comparisons of basal insulins were based solely on efficacy of glycaemic control. Many of the trials used in this analysis were treat-to-target, which minimize differences in HbA1c . In the NMAs, statistical significance was severely undermined by the wide credible intervals. Despite these limitations, point estimates of HbA1c were used to rank the insulins and formed the basis of NG17 guidance. This study queries whether such analyses should be used to make specific clinical recommendations.
© 2019 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Figures




References
-
- National Institute of Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management. 2015. Available at https://www.nice.org.uk/guidance/ng17/resources/type-1-diabetes-in-adult... Last accessed 8 February 2019.
-
- IQVIA . About the IQVIA CORE Diabetes Model. 2018. Available at http://www.core-diabetes.com/Index.aspx?Page=About Last accessed 8 February 2019.
-
- Baxter M, Hudson R, Mahon J, Bartlett C, Samyshkin Y, Alexiou D et al Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016; 33: 1575–1581. - PubMed
-
- National Institute of Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management. Appendices H‐U. 2015. Available at https://www.nice.org.uk/guidance/ng17/evidence/appendices-hu-pdf-435400240 Last accessed 8 February 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

