EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Cardiovascular MRI and CT in congenital heart disease
- PMID: 31730044
- PMCID: PMC6893312
- DOI: 10.1530/ERP-19-0048
EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Cardiovascular MRI and CT in congenital heart disease
Abstract
Cardiovascular MRI and CT are useful imaging modalities complimentary to echocardiography. This review article describes the common indications and consideration for the use of MRI and CT in the management of congenital heart disease.
Figures
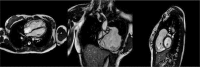
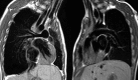





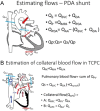
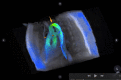


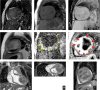
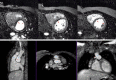
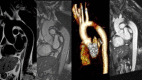

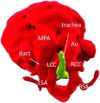
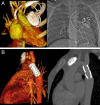

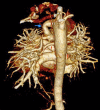

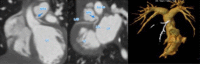
References
-
- Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM, Warnes CA, Kreutzer J, Geva T. Multimodality imaging guidelines for patients with repaired tetralogy of Fallot: a report from the American Society of Echocardiography: developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. Journal of the American Society of Echocardiography 2014. 111–141. (10.1016/j.echo.2013.11.009) - DOI - PubMed
-
- Cohen MS, Eidem BW, Cetta F, Fogel MA, Frommelt PC, Ganame J, Han BK, Kimball TR, Johnson RK, Mertens L, et al. Multimodality imaging guidelines of patients with transposition of the great arteries: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography 2016. 571–621. (10.1016/j.echo.2016.04.002) - DOI - PubMed
-
- Di Salvo G, Miller O, Babu Narayan S, Li W, Budts W, Valsangiacomo Buechel ER, Frigiola A, van den Bosch AE, Bonello B, Mertens L, et al. Imaging the adult with congenital heart disease: a multimodality imaging approach-position paper from the EACVI. European Heart Journal: Cardiovascular Imaging 2018. 1077–1098. (10.1093/ehjci/jey102) - DOI - PubMed
-
- Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. International Journal of Cardiovascular Imaging 2010. 57–64. (10.1007/s10554-009-9501-y) - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

