Incidence of acquired thrombotic thrombocytopenic purpura in Germany: a hospital level study
- PMID: 31730475
- PMCID: PMC6858672
- DOI: 10.1186/s13023-019-1240-0
Incidence of acquired thrombotic thrombocytopenic purpura in Germany: a hospital level study
Abstract
Background: Acquired thrombotic thrombocytopenic Purpura (aTTP) is a life-threatening ultra-orphan disease with a reported annual incidence between 1.5 and 6.0 cases per million in Europe and mainly affecting otherwise young and healthy adults aged 40 years on average. The goal of this study was to assess the incidence of aTTP in Germany.
Methods: A systematic review was performed to determine the published evidence on the aTTP epidemiology in Germany. To obtain additional evidence on the proportion of aTTP cases within the national Thrombotic Microangiopathy (TMA) population a hospital-level study was performed, using a retrospective data collection approach. Diagnosis of aTTP was confirmed if ADAMTS13 level were < 10% and/or the medical records explicitly mentioned aTTP diagnosis. The aggregated hospital data were then projected to the national level using logistic regression techniques.
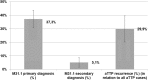
Results: The systematic literature search did not provide incidence estimates of aTTP in Germany. Eight centers (≈27% of the top 30 TMA hospitals) delivered data according to a predefined data collection form. On average (year 2014-2016) a total number of 172 aTTP episodes per year was projected (95% confidence interval [95%CI]: 132-212). The majority were newly diagnosed aTTP cases (n = 121; 95%CI: 105-129), and 51 were recurrent aTTP cases (95%CI: 27-84). The average annual projected incidence (year 2014-2016) of aTTP episodes was 2.10 per million inhabitants in Germany (95%CI: 1.60-2.58).
Conclusions: The determined annual incidence of newly diagnosed aTTP cases and the overall annual incidence of aTTP episodes in Germany confirm the ultra-orphan character of aTTP. An external validation against international registries (France, UK and USA) shows that our findings are quite comparable with those international incidence rates.
Keywords: Epidemiology; Germany; Incidence; Thrombotic microangiopathy; Thrombotic thrombocytopenic purpura.
Conflict of interest statement
WM: Prof. Miesbach reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, personal fees from Shir, outside the submitted work.
JM: Prof. Menne reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, personal fees from Sanofi Genzyme, personal fees from Alexion, outside the submitted work.
MB: Dr. Bommer reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, personal fees from Sanofi, personal fees from Alexion, outside the submitted work.
US: Dr. Schönermarck reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, personal fees from Alexion, outside the submitted work.
TF: Prof. Feldkamp reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, personal fees from Akari, personal fees from Alexion, outside the submitted work.
MN: Dr. Nitschke reports personal fees from Alexion, outside the submitted work.
TW: Prof. Westhoff has nothing to disclose.
FS: Dr. Seibert has nothing to disclose.
RW: Prof. Woitas has nothing to disclose.
RS: Dr. Sousa reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, outside the submitted work.
MW: Mr. Wolf reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, outside the submitted work.
SW: Dr. Walzer reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, outside the submitted work.
BS: Mr. Schwander reports personal fees from Ablynx a Sanofi Company, during the conduct of the study; personal fees from Ablynx a Sanofi Company, outside the submitted work.
Figures

References
-
- Goel R, King KE, Takemoto CM, Ness PM, Tobian AA. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451–1458. doi: 10.1111/trf.13586. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

