Intra-pancreatic tissue-derived mesenchymal stromal cells: a promising therapeutic potential with anti-inflammatory and pro-angiogenic profiles
- PMID: 31730488
- PMCID: PMC6858763
- DOI: 10.1186/s13287-019-1435-2
Intra-pancreatic tissue-derived mesenchymal stromal cells: a promising therapeutic potential with anti-inflammatory and pro-angiogenic profiles
Abstract
Background: Human pancreata contain many types of cells, such as endocrine islets, acinar, ductal, fat, and mesenchymal stromal cells (MSCs). MSCs are important and shown to have a promising therapeutic potential to treat various disease conditions.
Methods: We investigated intra-pancreatic tissue-derived (IPTD) MSCs isolated from tissue fractions that are routinely discarded during pancreatic islet isolation of human cadaveric donors. Furthermore, whether pro-angiogenic and anti-inflammatory properties of these cells could be enhanced was investigated.
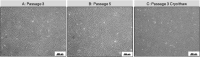
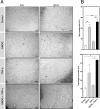
Results: IPTD-MSCs were expanded in GMP-compatible CMRL-1066 medium supplemented with 5% human platelet lysate (hPL). IPTD-MSCs were found to be highly pure, with > 95% positive for CD90, CD105, and CD73, and negative for CD45, CD34, CD14, and HLA-DR. Immunofluorescence staining of pancreas tissue demonstrated the presence of CD105+ cells in the vicinity of islets. IPTD-MSCs were capable of differentiation into adipocytes, chondrocytes, and osteoblasts in vitro, underscoring their multipotent features. When these cells were cultured in the presence of a low dose of TNF-α, gene expression of tumor necrosis factor alpha-stimulated gene-6 (TSG-6) was significantly increased, compared to control. In contrast, treating cells with dimethyloxallyl glycine (DMOG) (a prolyl 4-hydroxylase inhibitor) enhanced mRNA levels of nuclear factor erythroid 2-related factor 2 (NRF2) and vascular endothelial growth factor (VEGF). Interestingly, a combination of TNF-α and DMOG stimulated the optimal expression of all three genes in IPTD-MSCs. Conditioned medium of IPTD-MSCs treated with a combination of DMOG and TNF-α contained higher levels of pro-angiogenic (VEGF, IL-6, and IL-8) compared to controls, promoting angiogenesis of human endothelial cells in vitro. In contrast, levels of MCP-1, a pro-inflammatory cytokine, were reduced in the conditioned medium of IPTD-MSCs treated with a combination of DMOG and TNF-α.
Conclusions: The results demonstrate that IPTD-MSCs reside within the pancreas and can be separated as part of a standard islet-isolation protocol. These IPTD-MSCs can be expanded and potentiated ex vivo to enhance their anti-inflammatory and pro-angiogenic profiles. The fact that IPTD-MSCs are generated in a GMP-compatible procedure implicates a direct clinical application.
Keywords: Angiogenesis; Anti-inflammatory; Mesenchymal stromal cells; NRF2; TSG-6; Type 1 diabetes; VEGF.
Conflict of interest statement
BK, MQ, HTK, and IHA have patents pending related to this study. The other authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

