Global Trends in Marine Plankton Diversity across Kingdoms of Life
- PMID: 31730851
- PMCID: PMC6912166
- DOI: 10.1016/j.cell.2019.10.008
Global Trends in Marine Plankton Diversity across Kingdoms of Life
Abstract
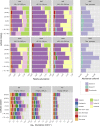
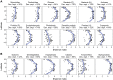
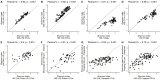
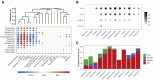
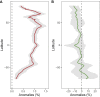
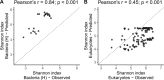
The ocean is home to myriad small planktonic organisms that underpin the functioning of marine ecosystems. However, their spatial patterns of diversity and the underlying drivers remain poorly known, precluding projections of their responses to global changes. Here we investigate the latitudinal gradients and global predictors of plankton diversity across archaea, bacteria, eukaryotes, and major virus clades using both molecular and imaging data from Tara Oceans. We show a decline of diversity for most planktonic groups toward the poles, mainly driven by decreasing ocean temperatures. Projections into the future suggest that severe warming of the surface ocean by the end of the 21st century could lead to tropicalization of the diversity of most planktonic groups in temperate and polar regions. These changes may have multiple consequences for marine ecosystem functioning and services and are expected to be particularly significant in key areas for carbon sequestration, fisheries, and marine conservation. VIDEO ABSTRACT.
Keywords: Tara Oceans; climate warming; high-throughput imaging; high-throughput sequencing; latitudinal diversity gradient; macroecology; plankton functional groups; temperature; trans-kingdom diversity.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Alberti A., Poulain J., Engelen S., Labadie K., Romac S., Ferrera I., Albini G., Aury J.-M., Belser C., Bertrand A., Genoscope Technical Team. Tara Oceans Consortium Coordinators Viral to metazoan marine plankton nucleotide sequences from the Tara Oceans expedition. Sci. Data. 2017;4:170093. - PMC - PubMed
-
- Algar A.C., Kharouba H.M., Young E.R., Kerr J.T. Predicting the future of species diversity: macroecological theory, climate change, and direct tests of alternative forecasting methods. Ecography. 2009;32:22–33.
-
- Aminot A., Kérouel R., Coverly S. Nutrients in Seawater Using Segmented Flow Analysis. In: Wurl O., editor. Practical Guidelines for the Analysis of Seawater. Taylor & Francis; 2009.

