Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype
- PMID: 31731024
- PMCID: PMC6960335
- DOI: 10.1016/j.actbio.2019.11.021
Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype
Abstract
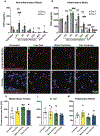
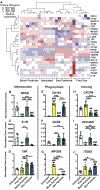
Monocyte-derived macrophages orchestrate tissue regeneration by homing to sites of injury, phagocytosing pathological debris, and stimulating other cell types to repair the tissue. Accordingly, monocytes have been investigated as a translational and potent source for cell therapy, but their utility has been hampered by their rapid acquisition of a pro-inflammatory phenotype in response to the inflammatory injury microenvironment. To overcome this problem, we designed a cell therapy strategy where monocytes are exogenously reprogrammed by intracellularly loading the cells with biodegradable microparticles containing an anti-inflammatory drug in order to modulate and maintain an anti-inflammatory phenotype over time. To test this concept, poly(lactic-co-glycolic) acid microparticles were loaded with the anti-inflammatory drug dexamethasone (Dex) and administered to primary human monocytes for four hours to facilitate phagocytic uptake. After removal of non-phagocytosed microparticles, microparticle-loaded monocytes differentiated into macrophages and stored the microparticles intracellularly for several weeks in vitro, releasing drug into the extracellular environment over time. Cells loaded with intracellular Dex microparticles showed decreased expression and secretion of inflammatory factors even in the presence of pro-inflammatory stimuli up to 7 days after microparticle uptake compared to untreated cells or cells loaded with blank microparticles, without interfering with phagocytosis of tissue debris. This study represents a new strategy for long-term maintenance of anti-inflammatory macrophage phenotype using a translational monocyte-based cell therapy strategy without the use of genetic modification. Because of the ubiquitous nature of monocyte-derived macrophage involvement in pathology and regeneration, this strategy holds potential as a treatment for a vast number of diseases and disorders. STATEMENT OF SIGNIFICANCE: We report a unique and translational strategy to overcome the challenges associated with monocyte- and macrophage-based cell therapies, in which the cells rapidly take on inflammatory phenotypes when administered to sites of injury. By intracellularly loading monocytes with drug-loaded microparticles prior to administration via phagocytosis, we were able to inhibit inflammation while preserving functional behaviors of human primary macrophages derived from those monocytes up to seven days later. To our knowledge, this study represents the first report of reprogramming macrophages to an anti-inflammatory phenotype without the use of genetic modification.
Keywords: Cell-microparticle interactions; Dexamethasone; Inflammation; Intracellular microparticles; Monocyte-derived macrophages; Phagocytosis.
Copyright © 2019. Published by Elsevier Ltd.
Figures



References
-
- Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Miskinyte G, Ge R, Ahlenius H, Lindvall O, Schwartz M, Kokaia Z, Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice, J. Neurosci 36 (2016) 4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016. - DOI - PMC - PubMed
-
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M, Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice., PLoS Med. 6 (2009) e1000113. doi: 10.1371/journal.pmed.1000113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

