MAMBO: Measuring ambulation, motor, and behavioral outcomes with post-stroke fluoxetine in Tanzania: Protocol of a phase II clinical trial
- PMID: 31731111
- PMCID: PMC6954330
- DOI: 10.1016/j.jns.2019.116563
MAMBO: Measuring ambulation, motor, and behavioral outcomes with post-stroke fluoxetine in Tanzania: Protocol of a phase II clinical trial
Abstract
Background: SSA has a high stroke incidence and post-stroke morbidity. An inexpensive pharmacological treatment for stroke recovery would be beneficial to patients in the region. Fluoxetine, currently on the World Health Organization Essential Medicines List, holds promise as a treatment for motor recovery after ischemic stroke, but its effectiveness is controversial and untested in this context in SSA.
Aim: To determine if fluoxetine 20 mg by mouth daily, given within 14 days of acute ischemic stroke, and taken for 90 days, is well-tolerated and safe with adequate adherence to justify a future randomized, controlled trial of fluoxetine in the United Republic of Tanzania.
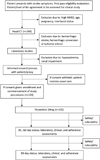
Methods: Open-label, phase II clinical trial enrolling up to 120 patients. Participants will be recruited from the Muhimbili National Hospital in Dar es Salaam, Tanzania, and followed for 90 days. The primary outcomes are: 1) safety, including serum sodium and hepatic enzyme levels; and 2) tolerability, as measured through study case report forms. The secondary outcomes are: 1) change in motor strength, as measured through the Fugl-Meyer Motor Scale; 2) adherence, as measured with electronic pill bottles; and 3) participant depressive symptom burden measured via standard questionnaires.
Conclusions: Expanding the evidence base for fluoxetine for Sub-Saharan African stroke survivors requires testing of its safety, tolerability, and adherence. Compared to prior studies in France and the United Kingdom, the patient characteristics, health infrastructure, and usual care for stroke recovery differ substantially in Tanzania. If fluoxetine reveals favorable endpoints, scale up of its use post-stroke is possible.
Keywords: Africa; Fluoxetine; Ischemic stroke; Motor recovery; Neuroplasticity; Phase II clinical trial; Stroke.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–1344. - PMC - PubMed
-
- Walker RW, Viney R, Green L, et al. Trends in stroke admissions to a Tanzanian hospital over four decades: a retrospective audit.Trop Med Int Health 2015;20:1290–1296. - PubMed
-
- Ezejimofor MC, Uthman OA, Maduka O, et al. Stroke survivors in Nigeria: A door-to-door prevalence survey from the Niger Delta region. J Neurol Sci 2017;372:262–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials