Open source silicon microprobes for high throughput neural recording
- PMID: 31731284
- PMCID: PMC7227378
- DOI: 10.1088/1741-2552/ab581a
Open source silicon microprobes for high throughput neural recording
Abstract
Objective: Microfabricated multielectrode arrays are widely used for high throughput recording of extracellular neural activity, which is transforming our understanding of brain function in health and disease. Currently there is a plethora of electrode-based tools being developed at higher education and research institutions. However, taking such tools from the initial research and development phase to widespread adoption by the neuroscience community is often hindered by several obstacles. The objective of this work is to describe the development, application, and open dissemination of silicon microprobes for recording neural activity in vivo.
Approach: We propose an open source dissemination platform as an alternative to commercialization. This framework promotes recording tools that are openly and inexpensively available to the community. The silicon microprobes are designed in house, but the fabrication and assembly processes are carried out by third party companies. This enables mass production, a key requirement for large-scale dissemination.
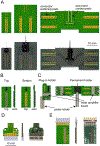
Main results: We demonstrate the operation of silicon microprobes containing up to 256 electrodes in conjunction with optical fibers for optogenetic manipulations or fiber photometry. These data provide new insights about the relationship between calcium activity and neural spiking activity. We also describe the current state of dissemination of these tools. A file repository of resources related to designing, using, and sharing these tools is maintained online.
Significance: This paper is likely to be a valuable resource for both current and prospective users, as well as developers of silicon microprobes. Based on their extensive usage by a number of labs including ours, these tools present a promising alternative to other types of electrode-based technologies aimed at high throughput recording in head-fixed animals. This work also demonstrates the importance of validating fiber photometry measurements with simultaneous electrophysiological recordings.
Conflict of interest statement
Disclosures
The authors declare the absence of any conflict of interest.
Figures









Similar articles
-
A microfabricated, 3D-sharpened silicon shuttle for insertion of flexible electrode arrays through dura mater into brain.J Neural Eng. 2019 Oct 29;16(6):066021. doi: 10.1088/1741-2552/ab2b2e. J Neural Eng. 2019. PMID: 31216526 Free PMC article.
-
A nanofabricated optoelectronic probe for manipulating and recording neural dynamics.J Neural Eng. 2018 Aug;15(4):046008. doi: 10.1088/1741-2552/aabc94. Epub 2018 Apr 9. J Neural Eng. 2018. PMID: 29629879 Free PMC article.
-
Low-cost and easy-fabrication lightweight drivable electrode array for multiple-regions electrophysiological recording in free-moving mice.J Neural Eng. 2022 Jan 24;19(1). doi: 10.1088/1741-2552/ac494e. J Neural Eng. 2022. PMID: 34996053
-
Design and manufacturing challenges of optogenetic neural interfaces: a review.J Neural Eng. 2017 Aug;14(4):041001. doi: 10.1088/1741-2552/aa7004. J Neural Eng. 2017. PMID: 28452331 Review.
-
Tools for probing local circuits: high-density silicon probes combined with optogenetics.Neuron. 2015 Apr 8;86(1):92-105. doi: 10.1016/j.neuron.2015.01.028. Neuron. 2015. PMID: 25856489 Free PMC article. Review.
Cited by
-
A silk-based self-adaptive flexible opto-electro neural probe.Microsyst Nanoeng. 2022 Nov 8;8:118. doi: 10.1038/s41378-022-00461-4. eCollection 2022. Microsyst Nanoeng. 2022. PMID: 36389054 Free PMC article.
-
Uncoordinated sleep replay across hemispheres in the zebra finch.Curr Biol. 2023 Nov 6;33(21):4704-4712.e3. doi: 10.1016/j.cub.2023.09.005. Epub 2023 Sep 26. Curr Biol. 2023. PMID: 37757833 Free PMC article.
-
Fabrication of Out-of-Plane High Channel Density Microelectrode Neural Array with 3D Recording and Stimulation Capabilities.J Microelectromech Syst. 2020 Aug;29(4):522-531. doi: 10.1109/jmems.2020.3004847. Epub 2020 Jul 2. J Microelectromech Syst. 2020. PMID: 39239464 Free PMC article.
-
Strong tuning for stereoscopic depth indicates orientation-specific recurrent circuitry in tree shrew V1.Curr Biol. 2022 Dec 19;32(24):5274-5284.e6. doi: 10.1016/j.cub.2022.10.063. Epub 2022 Nov 22. Curr Biol. 2022. PMID: 36417902 Free PMC article.
-
Context-dependent and dynamic functional influence of corticothalamic pathways to first- and higher-order visual thalamus.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):13066-13077. doi: 10.1073/pnas.2002080117. Epub 2020 May 27. Proc Natl Acad Sci U S A. 2020. PMID: 32461374 Free PMC article.
References
-
- Wise KD, Sodagar AM, Yao Y, Gulari MN, Perlin GE, and Najafi K, Microelectrodes, microelectronics, and implantable neural microsystems. Proceedings of the Ieee, 2008. 96(7): p. 1184–1202.
-
- Wise KD and Angell JB, Low-Capacitance Multielectrode Probe for Use in Extracellular Neurophysiology. Ieee Transactions on Biomedical Engineering, 1975. Bm22(3): p. 212–219. - PubMed
-
- Seymour JP and Kipke DR, Neural probe design for reduced tissue encapsulation in CNS. Biomaterials, 2007. 28(25): p. 3594–607. - PubMed
-
- Blanche TJ, Spacek MA, Hetke JF, and Swindale NV, Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. J Neurophysiol, 2005. 93(5): p. 2987–3000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
