Expression of Developmentally Important Axon Guidance Cues in the Adult Optic Chiasm
- PMID: 31731293
- PMCID: PMC6859889
- DOI: 10.1167/iovs.19-26732
Expression of Developmentally Important Axon Guidance Cues in the Adult Optic Chiasm
Abstract
Purpose: Regeneration of optic nerve axons after injury can be facilitated by several approaches, but misguidance at the optic chiasm is often observed. We characterized guidance cues in the embryonic visual system and adult optic chiasm before and after optic nerve crush (ONC) injury to better understand barriers to optic nerve regeneration in adults.
Methods: Radial glial (RC2/BLBP/Slit1), developmental (Pax2) and extracellular markers (CSPG: H2B/CS-56) were assessed in C57BL/6J mice by immunohistochemistry. RC2, BLBP, Slit1, and CSPG are known inhibitory guidance cues while Pax2 is a permissive guidance cue.
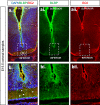
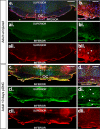
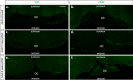
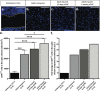
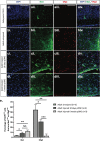
Results: At embryonic day 15.5 (E.15.5), RC2 and BLBP were identified superior to, and extending through, the optic chiasm. The optic chiasm was BLBP-ve in adult uninjured mice but BLBP+ve in adult mice 10 days after ONC injury. The reverse was true for RC2. Both BLBP and RC2 were absent in adult mice 6 weeks post-ONC. Slit1 was present in the optic chiasm midline and optic tracts in embryonic samples but was absent in uninjured adult tissue. Slit1 was observed superior to and at the midline of the optic chiasm 10 days post-ONC but absent 6 weeks after injury. Pax2 was expressed at the junction between the optic nerve and optic chiasm in embryonic brain tissue. In embryonic sections, CS-56 was observed at the junction between the optic chiasm and optic tract, and immediately superior to the optic chiasm. Both 2H6 and CS-56 staining was absent in uninjured and ONC-injured adult brains.
Conclusion: Differences in guidance cue expression during development, in adulthood and after injury may contribute to misguidance of regenerating RGC axons in the adult optic chiasm.
Figures





Similar articles
-
Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits.J Neurosci. 2000 Jul 1;20(13):4975-82. doi: 10.1523/JNEUROSCI.20-13-04975.2000. J Neurosci. 2000. PMID: 10864955 Free PMC article.
-
Retinal ganglion cell axon sorting at the optic chiasm requires dystroglycan.Dev Biol. 2018 Oct 15;442(2):210-219. doi: 10.1016/j.ydbio.2018.08.010. Epub 2018 Aug 24. Dev Biol. 2018. PMID: 30149005 Free PMC article.
-
Steerable-filter based quantification of axonal populations at the developing optic chiasm reveal significant defects in Slit2(-/-) as well as Slit1(-/-)Slit2(-/-) embryos.BMC Neurosci. 2013 Jan 15;14:9. doi: 10.1186/1471-2202-14-9. BMC Neurosci. 2013. PMID: 23320558 Free PMC article.
-
Guidance of retinal fibers in the optic chiasm.Perspect Dev Neurobiol. 1993;1(4):217-25. Perspect Dev Neurobiol. 1993. PMID: 8087546 Review.
-
Retinal axon guidance at the midline: Chiasmatic misrouting and consequences.Dev Neurobiol. 2017 Jul;77(7):844-860. doi: 10.1002/dneu.22473. Epub 2017 Feb 12. Dev Neurobiol. 2017. PMID: 27907266 Review.
Cited by
-
Retinal ganglion cell repopulation for vision restoration in optic neuropathy: a roadmap from the RReSTORe Consortium.Mol Neurodegener. 2023 Sep 21;18(1):64. doi: 10.1186/s13024-023-00655-y. Mol Neurodegener. 2023. PMID: 37735444 Free PMC article. Review.
-
Suppressing DNMT3a Alleviates the Intrinsic Epigenetic Barrier for Optic Nerve Regeneration and Restores Vision in Adult Mice.bioRxiv [Preprint]. 2023 Nov 23:2023.11.17.567614. doi: 10.1101/2023.11.17.567614. bioRxiv. 2023. PMID: 38014168 Free PMC article. Preprint.
-
Lhx2 promotes axon regeneration of adult retinal ganglion cells and rescues neurodegeneration in mouse models of glaucoma.Cell Rep Med. 2024 May 21;5(5):101554. doi: 10.1016/j.xcrm.2024.101554. Epub 2024 May 9. Cell Rep Med. 2024. PMID: 38729157 Free PMC article.
-
Optic Nerve Regeneration in Diabetic Retinopathy: Potentials and Challenges Ahead.Int J Mol Sci. 2023 Jan 11;24(2):1447. doi: 10.3390/ijms24021447. Int J Mol Sci. 2023. PMID: 36674963 Free PMC article. Review.
References
-
- Müller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

