Induced Pluripotent Stem Cells as Vasculature Forming Entities
- PMID: 31731464
- PMCID: PMC6912734
- DOI: 10.3390/jcm8111782
Induced Pluripotent Stem Cells as Vasculature Forming Entities
Abstract
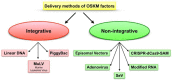
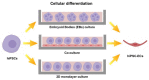
Tissue engineering (TE) pursues the ambitious goal to heal damaged tissues. One of the most successful TE approaches relies on the use of scaffolds specifically designed and fabricated to promote tissue growth. During regeneration the guidance of biological events may be essential to sustain vasculature neoformation inside the engineered scaffold. In this context, one of the most effective strategies includes the incorporation of vasculature forming cells, namely endothelial cells (EC), into engineered constructs. However, the most common EC sources currently available, intended as primary cells, are affected by several limitations that make them inappropriate to personalized medicine. Human induced Pluripotent Stem Cells (hiPSC), since the time of their discovery, represent an unprecedented opportunity for regenerative medicine applications. Unfortunately, human induced Pluripotent Stem Cells-Endothelial Cells (hiPSC-ECs) still display significant safety issues. In this work, we reviewed the most effective protocols to induce pluripotency, to generate cells displaying the endothelial phenotype and to perform an efficient and safe cell selection. We also provide noteworthy examples of both in vitro and in vivo applications of hiPSC-ECs in order to highlight their ability to form functional blood vessels. In conclusion, we propose hiPSC-ECs as the preferred source of endothelial cells currently available in the field of personalized regenerative medicine.
Keywords: angiogenesis; from bench to bedside; induced pluripotent stem cells; tissue engineering; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Barabaschi G.D.G., Manoharan V. Engineering Mineralized and Load Bearing Tissues. Adv. Exp. Med. Biol. 2015;881:79–94. - PubMed
-
- Baranski J.D., Chaturvedi R.R., Stevens K.R., Eyckmans J., Carvalho B., Solorzano R.D., Yang M.T., Miller J.S., Bhatia S.N., Chen C.S. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA. 2013;110:7586–7591. doi: 10.1073/pnas.1217796110. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

