Role of Receptor Profiling for Personalized Therapy in a Patient with a Growth Hormone-Secreting Macroadenoma Resistant to First-Generation Somatostatin Analogues
- PMID: 31731613
- PMCID: PMC6963904
- DOI: 10.3390/jpm9040048
Role of Receptor Profiling for Personalized Therapy in a Patient with a Growth Hormone-Secreting Macroadenoma Resistant to First-Generation Somatostatin Analogues
Abstract
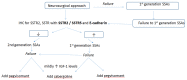
Background: Acromegaly is almost always caused by a pituitary adenoma and is associated with high morbidity and mortality when uncontrolled. Trans-sphenoidal removal of the adenoma is the mainstay of therapy, but fails to control the disease in a significant number of patients who require further treatment. Somatostatin analogues (SSAs) as monotherapy or in combination with growth hormone (GH)-receptor antagonists and/or dopamine agonists are used either alone or in combination following surgical failure to achieve disease control. The use of specific biomarkers may help to individualize the therapeutic plan after surgical failure and direct towards a more personalized approach.
Methods: We report a 41-year-old man with acromegaly and residual disease after repeated surgery that was resistant to first-generation SSAs.
Results: Biochemical and tumor control were achieved following the administration of a second-generation SSA, pasireotide, combined with pegvisomant, both at maximal doses and along with cabergoline. Histology specimens showed a sparsely-granulated GH-immunostaining pituitary adenoma with intense positivity for somatostatin receptors 2 and 5 and low levels of E-cadherin.
Conclusion: Personalized medical therapy guided by currently available biomarkers, such as immunohistochemically-characterized receptor profiling or adhesion molecules, resulted in controlled insulin-like growth factor-1 (IGF-1) and GH levels and symptom alleviation following the combination of three drug-classes.
Keywords: acromegaly; e-cadherin; personalized treatment; resistant acromegaly; somatostatin analogue; somatostatin receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dal J., Leisner M.Z., Hermansen K., Farkas D.K., Bengtsen M., Kistorp C., Nielsen E.H., Andersen M., Feldt-Rasmussen U., Dekkers O.M., et al. Cancer Incidence in Patients with Acromegaly: A Cohort Study and Meta-Analysis of the Literature. J. Clin. Endocrinol. Metab. 2018;103:2182–2188. doi: 10.1210/jc.2017-02457. - DOI - PubMed
-
- French Acromegaly Registry Group. Maione L., Brue T., Beckers A., Delemer B., Petrossians P., Borson-Chazot F., Chabre O., François P., Bertherat J., et al. Changes in the management and comorbidities of acromegaly over three decades: The French Acromegaly Registry. Eur. J. Endocrinol. 2017;176:645–655. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

