Ullmann Reactions of Carbon Nanotubes-Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry
- PMID: 31731640
- PMCID: PMC6915440
- DOI: 10.3390/nano9111619
Ullmann Reactions of Carbon Nanotubes-Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry
Abstract
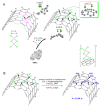
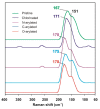
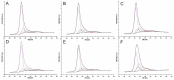
We demonstrate Ullmann-type reactions as novel and advantageous functionalization of carbon nanotubes (CNTs) toward tunable surface chemistry. The functionalization routes comprise O-, N-, and C-arylation of chlorinated CNTs. We confirm the versatility and efficiency of the reaction allowing functionalization degrees up to 3.5 mmol g-1 by applying both various nanotube substrates, i.e., single-wall (SWCNTs) and multi-wall CNTs (MWCNTs) of various chirality, geometry, and morphology as well as diverse Ullmann-type reagents: phenol, aniline, and iodobenzene. The reactivity of nanotubes was correlatable with the nanotube diameter and morphology revealing SWCNTs as the most reactive representatives. We have determined the optimized conditions of this two-step synthetic protocol as: (1) chlorination using iodine trichloride (ICl3), and (2) Ullmann-type reaction in the presence of: copper(I) iodide (CuI), 1,10-phenanthroline as chelating agent and caesium carbonate (Cs2CO3) as base. We have analyzed functionalized CNTs using a variety of techniques, i.e., scanning and transmission electron microscopy, energy dispersive spectroscopy, thermogravimetry, comprehensive Raman spectroscopy, and X-ray photoelectron spectroscopy. The analyses confirmed the purely covalent nature of those modifications at all stages. Eventually, we have proved the elaborated protocol as exceptionally tunable since it enabled us: (a) to synthesize superhydrophilic films from-the intrinsically hydrophobic-vertically aligned MWCNT arrays and (b) to produce printable highly electroconductive pastes of enhanced characteristics-as compared for non-modified and otherwise modified MWCNTs-for textronics.
Keywords: Ullmann reaction; carbon nanotubes; chlorination; electroconductive coatings; functionalization; hydrophilization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Covalent Functionalization of Multi-Walled Carbon Nanotubes Surface via Chemical Treatment.J Nanosci Nanotechnol. 2017 Apr;17(4):2463-470. doi: 10.1166/jnn.2017.13311. J Nanosci Nanotechnol. 2017. PMID: 29648764
-
Conformal atomic layer deposition of alumina on millimeter tall, vertically-aligned carbon nanotube arrays.ACS Appl Mater Interfaces. 2014 Nov 12;6(21):19135-43. doi: 10.1021/am505107s. Epub 2014 Oct 15. ACS Appl Mater Interfaces. 2014. PMID: 25275708
-
Oxidised carbon nanotubes as dual-domain synergetic stabilizers in electroconductive carbon nanotube flexible coatings.RSC Adv. 2018 Aug 31;8(54):30712-30716. doi: 10.1039/c8ra05902k. eCollection 2018 Aug 30. RSC Adv. 2018. PMID: 35548761 Free PMC article.
-
Nanotube Functionalization: Investigation, Methods and Demonstrated Applications.Materials (Basel). 2022 Aug 5;15(15):5386. doi: 10.3390/ma15155386. Materials (Basel). 2022. PMID: 35955321 Free PMC article. Review.
-
Functionalization of carbon nanotubes: manufacturing techniques and properties of customized nanocomponents for molecular-level technology.Recent Pat Nanotechnol. 2009;3(2):154-61. doi: 10.2174/187221009788490013. Recent Pat Nanotechnol. 2009. PMID: 19519597 Review.
Cited by
-
Functionalization of Carbon Nanotubes Surface by Aryl Groups: A Review.Nanomaterials (Basel). 2023 May 13;13(10):1630. doi: 10.3390/nano13101630. Nanomaterials (Basel). 2023. PMID: 37242046 Free PMC article. Review.
-
Multifunctional Conductive Paths Obtained by Laser Processing of Non-Conductive Carbon Nanotube/Polypropylene Composites.Nanomaterials (Basel). 2021 Feb 28;11(3):604. doi: 10.3390/nano11030604. Nanomaterials (Basel). 2021. PMID: 33670969 Free PMC article.
-
Carbon nanotube materials for electrocardiography.RSC Adv. 2021 Jan 14;11(5):3020-3042. doi: 10.1039/d0ra08679g. eCollection 2021 Jan 11. RSC Adv. 2021. PMID: 35424207 Free PMC article. Review.
-
Semi-Continuous Heterophase Polymerization to Synthesize Poly(methacrylic acid)-Based Nanocomposites for Drug Delivery.Polymers (Basel). 2022 Mar 16;14(6):1195. doi: 10.3390/polym14061195. Polymers (Basel). 2022. PMID: 35335527 Free PMC article.
References
-
- Schnorr J.M., Swager T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011;23:646–657. doi: 10.1021/cm102406h. - DOI
-
- Treacy M.M.J., Ebbesen T.W., Gibson J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature. 1996;381:678–680. doi: 10.1038/381678a0. - DOI
-
- Khandoker N., Hawkins S.C., Ibrahim R., Huynh C.P., Deng F. Tensile Strength of Spinnable Multiwall Carbon Nanotubes. Procedia Eng. 2011;10:2572–2578. doi: 10.1016/j.proeng.2011.04.424. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

