Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling
- PMID: 31731716
- PMCID: PMC6912538
- DOI: 10.3390/antiox8110557
Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling
Abstract
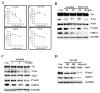
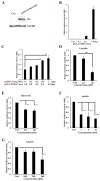
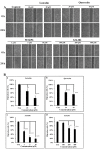
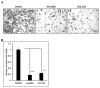
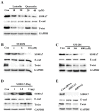
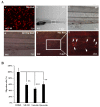
Flavonoids are well-known antioxidants and have shown the ability to prevent tumor formation and recurrence. Especially in dietary flavonoids, they have provided convenience and consistence of intake for long-term prevention of tumor formation. Previous reports suggested that S100 calcium-binding protein A7 (S100A7) might activate epithelial-mesenchymal transition (EMT) signaling and promote the metastasis of tumor cells; however, the regulatory signaling was unclear. In this study, we found that S100A7 was highly expressed in cancer cells and could be reduced by luteolin (Lu) and quercetin (Qu) through Src/Stat3 signaling. We found that the protein levels of S100A7, phosphorylated Src (p-Src), and p-Stat3 were increased in A431-III cells. Flavonoids Lu and Qu reduce protein levels of p-Src, p-Stat3 and S100A7 in A431-III cells. Treatment of A431-III cells with Src inhibitor SU6656 and Stat3 inhibitor S3I-201 also reduced the protein levels of S100A7. Transactivation activity of 5'-upstream regions of S100A7 was activated by Stat3 but was reduced by treatment with Lu, Qu, SU6656 and S3I-201. The treatment also reduced the migratory and invasive abilities of A431-III cells. In a further analysis of EMT markers, the protein level of E-cad increased and that of Twist decreased after treatment with the inhibitors and flavonoids. Overexpression of S100A7 decreased the protein level of E-cad and increased the Twist level, whereas knockdown of S100A7 had the opposite effects. Treatment with S3I-201, Lu and Qu, compared to the control, were found to decrease metastasis of tumor cells in zebrafish larvae. These results suggest that Lu and Qu may inhibit Src/Stat3/S100A7 signaling to reduce tumorigenesis of cancer cells.
Keywords: S100A7; Src; Stat3; luteolin; metastasis; quercetin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor.Br J Pharmacol. 1999 Nov;128(5):999-1010. doi: 10.1038/sj.bjp.0702879. Br J Pharmacol. 1999. PMID: 10556937 Free PMC article.
-
Impact of flavonoids on matrix metalloproteinase secretion and invadopodia formation in highly invasive A431-III cancer cells.PLoS One. 2013 Aug 21;8(8):e71903. doi: 10.1371/journal.pone.0071903. eCollection 2013. PLoS One. 2013. PMID: 23991004 Free PMC article.
-
Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells.Cancer Sci. 2011 Oct;102(10):1829-39. doi: 10.1111/j.1349-7006.2011.02035.x. Epub 2011 Aug 10. Cancer Sci. 2011. PMID: 21752154
-
Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells.Anticancer Res. 2002 May-Jun;22(3):1615-27. Anticancer Res. 2002. PMID: 12168845
-
Dietary flavonoids, luteolin and quercetin, inhibit invasion of cervical cancer by reduction of UBE2S through epithelial-mesenchymal transition signaling.Food Funct. 2017 Apr 19;8(4):1558-1568. doi: 10.1039/c6fo00551a. Food Funct. 2017. PMID: 28277581
Cited by
-
Bufalin inhibits epithelial-mesenchymal transition and increases radiosensitivity of non-small cell lung cancer via inhibition of the Src signaling.J Thorac Dis. 2023 Jan 31;15(1):123-134. doi: 10.21037/jtd-22-1859. Epub 2023 Jan 16. J Thorac Dis. 2023. PMID: 36794138 Free PMC article.
-
Insulin/IGF Axis and the Receptor for Advanced Glycation End Products: Role in Meta-inflammation and Potential in Cancer Therapy.Endocr Rev. 2023 Jul 11;44(4):693-723. doi: 10.1210/endrev/bnad005. Endocr Rev. 2023. PMID: 36869790 Free PMC article. Review.
-
Recent advances in valorization of citrus fruits processing waste: a way forward towards environmental sustainability.Food Sci Biotechnol. 2021 Oct 7;30(13):1601-1626. doi: 10.1007/s10068-021-00984-y. eCollection 2021 Dec. Food Sci Biotechnol. 2021. PMID: 34925937 Free PMC article. Review.
-
Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis.Cancers (Basel). 2020 Jul 21;12(7):1985. doi: 10.3390/cancers12071985. Cancers (Basel). 2020. PMID: 32708138 Free PMC article. Review.
-
Co-administration of Chrysin and Luteolin with Cisplatin and Topotecan Exhibits a Variable Therapeutic Value in Human Cancer Cells, HeLa.ACS Omega. 2023 Oct 27;8(44):41204-41213. doi: 10.1021/acsomega.3c04443. eCollection 2023 Nov 7. ACS Omega. 2023. PMID: 37970041 Free PMC article.
References
-
- Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

