MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness
- PMID: 31731750
- PMCID: PMC6888443
- DOI: 10.3390/ijms20225742
MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness
Abstract
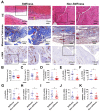
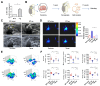
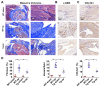
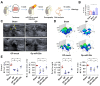
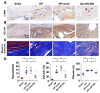
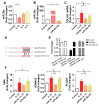
Rotator cuff lesion with shoulder stiffness is a major cause of shoulder pain and motionlessness. Subacromial bursa fibrosis is a prominent pathological feature of the shoulder disorder. MicroRNA-29a (miR-29a) regulates fibrosis in various tissues; however, the miR-29a action to subacromial bursa fibrosis remains elusive. Here, we reveal that subacromial synovium in patients with rotator cuff tear with shoulder stiffness showed severe fibrosis, hypertrophy, and hyperangiogenesis histopathology along with significant increases in fibrotic matrices collagen (COL) 1A1, 3A1, and 4A1 and inflammatory cytokines, whereas miR-29a expression was downregulated. Supraspinatus and infraspinatus tenotomy-injured shoulders in transgenic mice overexpressing miR-29a showed mild swelling, vascularization, fibrosis, and regular gait profiles as compared to severe rotator cuff damage in wild-type mice. Treatment with miR-29a precursor compromised COL3A1 production and hypervascularization in injured shoulders. In vitro, gain of miR-29a function attenuated COL3A1 expression through binding to the 3'-untranslated region (3'-UTR) of COL3A1 in inflamed tenocytes, whereas silencing miR-29a increased the matrix expression. Taken together, miR-29a loss is correlated with subacromial bursa inflammation and fibrosis in rotator cuff tear with shoulder stiffness. miR-29a repressed subacromial bursa fibrosis through directly targeting COL3A1 mRNA, improving rotator cuff integrity and shoulder function. Collective analysis offers a new insight into the molecular mechanism underlying rotator cuff tear with shoulder stiffness. This study also highlights the remedial potential of miR-29a precursor for alleviating the shoulder disorder.
Keywords: fibrosis; miR-29a; shoulder stiffness; subacromial bursa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Immunolocalization of cytokines and growth factors in subacromial bursa of rotator cuff tear patients.Kobe J Med Sci. 2001 Feb;47(1):25-34. Kobe J Med Sci. 2001. PMID: 11565192
-
Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases.J Orthop Res. 1998 Sep;16(5):618-21. doi: 10.1002/jor.1100160515. J Orthop Res. 1998. PMID: 9820287
-
The subacromial bursa modulates tendon healing after rotator cuff injury in rats.Sci Transl Med. 2024 Apr 24;16(744):eadd8273. doi: 10.1126/scitranslmed.add8273. Epub 2024 Apr 24. Sci Transl Med. 2024. PMID: 38657023 Free PMC article.
-
The Subacromial Bursa: Current Concepts Review.JBJS Rev. 2021 Nov 10;9(11). doi: 10.2106/JBJS.RVW.21.00110. JBJS Rev. 2021. PMID: 34757977 Review.
-
Rotator cuff lesions with shoulder stiffness: updated pathomechanisms and management.Chang Gung Med J. 2011 Jul-Aug;34(4):331-40. Chang Gung Med J. 2011. PMID: 21880187 Review.
Cited by
-
MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6.Int J Mol Sci. 2023 May 23;24(11):9158. doi: 10.3390/ijms24119158. Int J Mol Sci. 2023. PMID: 37298111 Free PMC article.
-
si-Tgfbr1-loading liposomes inhibit shoulder capsule fibrosis via mimicking the protective function of exosomes from patients with adhesive capsulitis.Biomater Res. 2022 Aug 19;26(1):39. doi: 10.1186/s40824-022-00286-2. Biomater Res. 2022. PMID: 35986376 Free PMC article.
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research.Cells. 2022 Feb 14;11(4):663. doi: 10.3390/cells11040663. Cells. 2022. PMID: 35203311 Free PMC article. Review.
-
The role of MicroRNAs in tendon injury, repair, and related tissue engineering.Biomaterials. 2021 Oct;277:121083. doi: 10.1016/j.biomaterials.2021.121083. Epub 2021 Aug 26. Biomaterials. 2021. PMID: 34488121 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

