Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer
- PMID: 31732494
- PMCID: PMC6954326
- DOI: 10.1158/2159-8290.CD-19-0980
Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer
Abstract
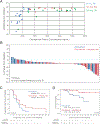
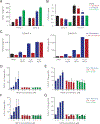
Adenosine mediates immunosuppression within the tumor microenvironment through triggering adenosine 2A receptors (A2AR) on immune cells. To determine whether this pathway could be targeted as an immunotherapy, we performed a phase I clinical trial with a small-molecule A2AR antagonist. We find that this molecule can safely block adenosine signaling in vivo. In a cohort of 68 patients with renal cell cancer (RCC), we also observe clinical responses alone and in combination with an anti-PD-L1 antibody, including subjects who had progressed on PD-1/PD-L1 inhibitors. Durable clinical benefit is associated with increased recruitment of CD8+ T cells into the tumor. Treatment can also broaden the circulating T-cell repertoire. Clinical responses are associated with an adenosine-regulated gene-expression signature in pretreatment tumor biopsies. A2AR signaling, therefore, represents a targetable immune checkpoint distinct from PD-1/PD-L1 that restricts antitumor immunity. SIGNIFICANCE: This first-in-human study of an A2AR antagonist for cancer treatment establishes the safety and feasibility of targeting this pathway by demonstrating antitumor activity with single-agent and anti-PD-L1 combination therapy in patients with refractory RCC. Responding patients possess an adenosine-regulated gene-expression signature in pretreatment tumor biopsies.See related commentary by Sitkovsky, p. 16.This article is highlighted in the In This Issue feature, p. 1.
©2019 American Association for Cancer Research.
Figures


Comment in
-
Lessons from the A2A Adenosine Receptor Antagonist-Enabled Tumor Regression and Survival in Patients with Treatment-Refractory Renal Cell Cancer.Cancer Discov. 2020 Jan;10(1):16-19. doi: 10.1158/2159-8290.CD-19-1280. Cancer Discov. 2020. PMID: 31919119
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

