Clinical Evolution of Epithelial-Mesenchymal Transition in Human Carcinomas
- PMID: 31732654
- PMCID: PMC8170833
- DOI: 10.1158/0008-5472.CAN-18-3539
Clinical Evolution of Epithelial-Mesenchymal Transition in Human Carcinomas
Abstract
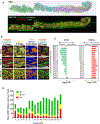
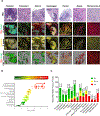
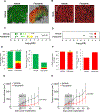
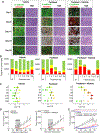
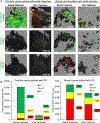
The significance of the phenotypic plasticity afforded by epithelial-mesenchymal transition (EMT) for cancer progression and drug resistance remains to be fully elucidated in the clinic. We evaluated epithelial-mesenchymal phenotypic characteristics across a range of tumor histologies using a validated, high-resolution digital microscopic immunofluorescence assay (IFA) that incorporates β-catenin detection and cellular morphology to delineate carcinoma cells from stromal fibroblasts and that quantitates the individual and colocalized expression of the epithelial marker E-cadherin (E) and the mesenchymal marker vimentin (V) at subcellular resolution ("EMT-IFA"). We report the discovery of β-catenin+ cancer cells that coexpress E-cadherin and vimentin in core-needle biopsies from patients with various advanced metastatic carcinomas, wherein these cells are transitioning between strongly epithelial and strongly mesenchymal-like phenotypes. Treatment of carcinoma models with anticancer drugs that differ in their mechanism of action (the tyrosine kinase inhibitor pazopanib in MKN45 gastric carcinoma xenografts and the combination of tubulin-targeting agent paclitaxel with the BCR-ABL inhibitor nilotinib in MDA-MB-468 breast cancer xenografts) caused changes in the tumor epithelial-mesenchymal character. Moreover, the appearance of partial EMT or mesenchymal-like carcinoma cells in MDA-MB-468 tumors treated with the paclitaxel-nilotinib combination resulted in upregulation of cancer stem cell (CSC) markers and susceptibility to FAK inhibitor. A metastatic prostate cancer patient treated with the PARP inhibitor talazoparib exhibited similar CSC marker upregulation. Therefore, the phenotypic plasticity conferred on carcinoma cells by EMT allows for rapid adaptation to cytotoxic or molecularly targeted therapy and could create a form of acquired drug resistance that is transient in nature. SIGNIFICANCE: Despite the role of EMT in metastasis and drug resistance, no standardized assessment of EMT phenotypic heterogeneity in human carcinomas exists; the EMT-IFA allows for clinical monitoring of tumor adaptation to therapy.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016;35:645–54. - PubMed
-
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97–110. - PubMed
-
- Bastid J. EMT in carcinoma progression and dissemination: facts, unanswered questions, and clinical considerations. Cancer Metastasis Rev 2012;31:277–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

