Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells
- PMID: 31732721
- PMCID: PMC7275645
- DOI: 10.1038/s41596-019-0220-0
Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells
Abstract
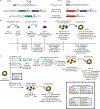
Alveolar epithelial type II cells (AEC2s) are the facultative progenitors of lung alveoli and serve as the surfactant-producing cells of air-breathing organisms. Although primary human AEC2s are difficult to maintain stably in cell cultures, recent advances have facilitated the derivation of AEC2-like cells from human pluripotent stem cells (hPSCs) in vitro. Here, we provide a detailed protocol for the directed differentiation of hPSCs into self-renewing AEC2-like cells that can be maintained for up to 1 year in culture as epithelial-only spheres without the need for supporting mesenchymal feeder cells. The month-long protocol requires recapitulation of the sequence of milestones associated with in vivo development of the distal lung, beginning with differentiation of cells into anterior foregut endoderm, which is followed by their lineage specification into NKX2-1+ lung progenitors and then distal/alveolar differentiation to produce progeny that express transcripts and possess functional properties associated with AEC2s.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures






References
-
- Mason RJ & Williams MC Type II alveolar cell. Defender of the alveolus. Am. Rev. Respir. Dis. 115, 81–91 (1977). - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL128172/HL/NHLBI NIH HHS/United States
- U01 TR001810/TR/NCATS NIH HHS/United States
- U01 HL099997/HL/NHLBI NIH HHS/United States
- R24 HL123828/HL/NHLBI NIH HHS/United States
- TL1 TR001410/TR/NCATS NIH HHS/United States
- U01 HL134745/HL/NHLBI NIH HHS/United States
- R01 HL139799/HL/NHLBI NIH HHS/United States
- R01 HL095993/HL/NHLBI NIH HHS/United States
- F31 HL134274/HL/NHLBI NIH HHS/United States
- R01 HL122442/HL/NHLBI NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- U01 HL134766/HL/NHLBI NIH HHS/United States
- U01 HL148692/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

